Immunogenicity of a killed Leishmania vaccine with saponin adjuvant in dogs
- PMID: 17913311
- PMCID: PMC7115514
- DOI: 10.1016/j.vaccine.2007.08.009
Immunogenicity of a killed Leishmania vaccine with saponin adjuvant in dogs
Abstract
Cellular and humoral immune responses of dogs to a candidate vaccine, composed of Leishmania braziliensis promastigote protein plus saponin as adjuvant, have been investigated as a pre-requisite to understanding the mechanisms of immunogenicity against canine visceral leishmaniasis (CVL). The candidate vaccine elicited strong antigenicity related to the increases of anti-Leishmania IgG isotypes, together with higher levels of lymphocytes, particularly of circulating CD8(+) T-lymphocytes and Leishmania chagasi antigen-specific CD8(+) T-lymphocytes. As indicated by the intense cell proliferation and increased nitric oxide production during in vitro stimulation by L. chagasi soluble antigens, the candidate vaccine elicited an immune activation status potentially compatible with effective control of the etiological agent of CVL.
Figures
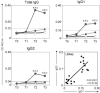
 ); LB (killed L. braziliensis vaccine;
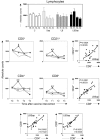
); LB (killed L. braziliensis vaccine;  ), LBSap (killed L. braziliensis vaccine plus saponin; ■)] and middle left panels [C (control; □); Sap (saponin; ○); LB (killed L. braziliensis vaccine; ■); LBSap (killed L. braziliensis vaccine plus saponin; ●)]: the x-axis displays the times at which the assays were conducted (T0: prior to the first dose; T1: 15 days after the first dose; T2: 15 days after the second dose; and T3: 15 days after the third dose) and the y-axis represents the mean values (with standard deviations in the upper panel) of the absolute counts of circulating lymphocytes (upper panel), and of CD5+, CD21+, CD4+ and CD8+ cells (middle left panels). Significant differences (P < 0.05) between the LBSap group and the control C and Sap groups are indicated, respectively, by the letters a, b. The middle right panels and the lower panels show the correlations between circulating lymphocytes in the LBSap group and the Pearson's correlation indexes (r) at P < 0.05 are shown in figure.
), LBSap (killed L. braziliensis vaccine plus saponin; ■)] and middle left panels [C (control; □); Sap (saponin; ○); LB (killed L. braziliensis vaccine; ■); LBSap (killed L. braziliensis vaccine plus saponin; ●)]: the x-axis displays the times at which the assays were conducted (T0: prior to the first dose; T1: 15 days after the first dose; T2: 15 days after the second dose; and T3: 15 days after the third dose) and the y-axis represents the mean values (with standard deviations in the upper panel) of the absolute counts of circulating lymphocytes (upper panel), and of CD5+, CD21+, CD4+ and CD8+ cells (middle left panels). Significant differences (P < 0.05) between the LBSap group and the control C and Sap groups are indicated, respectively, by the letters a, b. The middle right panels and the lower panels show the correlations between circulating lymphocytes in the LBSap group and the Pearson's correlation indexes (r) at P < 0.05 are shown in figure.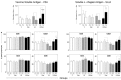
 ); LB (killed L. braziliensis vaccine;
); LB (killed L. braziliensis vaccine;  ); LBSap (killed L. braziliensis vaccine plus saponin; ■). The results are expressed as the mean frequencies of CD5+, CD21+, CD4+ and CD8+ cells in the non-stimulated cultures (CC; controls) and in the stimulated cultures (SC). Significant differences (P < 0.05) between values measured at T0 (before the first dose) and T3 (15 days after the third dose) are indicated by connecting lines, and between the LBSap and the control C, Sap, and LB groups at T3 are represented by the letters a, b, and c, respectively.
); LBSap (killed L. braziliensis vaccine plus saponin; ■). The results are expressed as the mean frequencies of CD5+, CD21+, CD4+ and CD8+ cells in the non-stimulated cultures (CC; controls) and in the stimulated cultures (SC). Significant differences (P < 0.05) between values measured at T0 (before the first dose) and T3 (15 days after the third dose) are indicated by connecting lines, and between the LBSap and the control C, Sap, and LB groups at T3 are represented by the letters a, b, and c, respectively.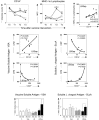
 ); LB (killed L. braziliensis vaccine;
); LB (killed L. braziliensis vaccine;  ); LBSap (killed L. braziliensis vaccine plus saponin; ■). Significant differences (P < 0.05) between at T0 plus T3 are indicated by connecting lines.
); LBSap (killed L. braziliensis vaccine plus saponin; ■). Significant differences (P < 0.05) between at T0 plus T3 are indicated by connecting lines.
References
-
- Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. - PubMed
-
- Evans T.G., Vasconcelos I.A., Lima J.W., Teixeira J.M., McAullife I.T., Lopes U.G. Canine visceral leishmaniasis in northeast Brazil: assessment of serodiagnostic methods. Am J Trop Med Hyg. 1990;42:118–123. - PubMed
-
- Tesh R.B. Control of zoonotic visceral leishmaniasis: is it time to change strategies? Am J Trop Med Hyg. 1995;52:287–292. - PubMed
-
- Braga M.D., Coelho I.C., Pompeu M.M., Evans T.G., MacAullife I.T., Teixeira M.J. Controle do calazar canino: comparação dos resultados de um programa de eliminação rápida de cães sororreagentes por ensaio imuno-enzimático com outro de eliminação tardia de cães sororreagentes por teste de imunofluorescência indireta de eluato de papel filtro. Rev Soc Bras Med Trop. 1998;31:419–424. - PubMed
-
- Franca-Silva J.C., da Costa R.T., Siqueira A.M., hado-Coelho G.L., da Costa C.A., Mayrink W. Epidemiology of canine visceral leishmaniosis in the endemic area of Montes Claros Municipality, Minas Gerais State, Brazil. Vet Parasitol. 2003;111:161–173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

