Sensor complexes regulating two-component signal transduction
- PMID: 17913492
- PMCID: PMC2175030
- DOI: 10.1016/j.sbi.2007.08.019
Sensor complexes regulating two-component signal transduction
Abstract
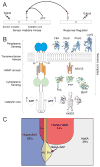
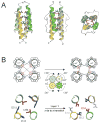
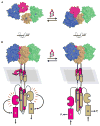
Two-component signal transduction systems consisting of a sensor histidine kinase and a response regulator/transcription factor interpret a multitude of environmental and cellular signals and coordinate the expression of a wide array of genes in bacteria. Signal recognition by sensor histidine kinases is the province of a sensor complex consisting of several protein domains that together serve to augment or attenuate the activity of the histidine kinase and thereby of gene expression. Recent investigations have shown the diverse strategies bacteria use to assemble protein domains into the sensor complexes to accomplish signaling. Structural studies of such domains are leading to an understanding of the mechanisms by which sensor complexes recognize signals and regulate kinase activity.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

