Ethanol teratogenesis in Japanese medaka: effects at the cellular level
- PMID: 17913529
- PMCID: PMC2220156
- DOI: 10.1016/j.cbpb.2007.09.008
Ethanol teratogenesis in Japanese medaka: effects at the cellular level
Abstract
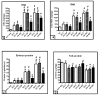
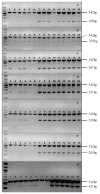
The adverse effects of alcohol on the developing humans represent a spectrum of structural and neurobehavioral abnormalities, most appropriately termed as fetal alcohol spectrum disorder (FASD). The mechanism by which ethanol induces FASD is unknown. Human studies of FASD are very limited due to ethical constraints; however, several animal models from nematodes to mammals are utilized to understand the molecular mechanism of this disorder. We have used Japanese medaka (Oryzias latipes) embryo-larval development as a unique non-mammalian model to study the molecular mechanism of FASD. Fertilized medaka eggs were exposed to ethanol (0-400 mM) for 48 h post fertilization (hpf) and then maintained in regular embryo rearing medium without ethanol. Viable embryos were harvested on 0, 2, 4 and 6 day post fertilization (dpf) and analyzed for DNA, RNA and protein contents of the embryos. By applying semi-quantitative RT-PCR (rRT-PCR) and quantitative real-time RT-PCR (qRT-PCR), RNA samples were further analyzed for seven transcription factors, emx2, en2, iro3, otx2, shh, wnt1 and zic5 which are expressed in the neural tube of medaka embryo during early phase of development. RNA and protein contents of the embryos were significantly reduced by ethanol at 400 mM dose on 4 and 6 dpf compared to the control (no ethanol), and 100 mM ethanol treated embryos. However, significant reduction of DNA was observed only in 4 dpf embryos. Total protein contents of yolk remained unaltered after ethanol treatment. Expression pattern of emx2, en2, iro3, otx2, shh, wnt1, and zic5 mRNAs were found to be developmentally regulated, however, remained unaltered after ethanol treatment. It is therefore concluded that alteration of nucleic acid and protein contents of medaka embryo by ethanol could be used as an indicator of embryonic growth retardation which might be the result of disruption of specific gene function during development.
Figures
Similar articles
-
Disruption of circulation by ethanol promotes fetal alcohol spectrum disorder (FASD) in medaka (Oryzias latipes) embryogenesis.Comp Biochem Physiol C Toxicol Pharmacol. 2008 Sep;148(3):273-80. doi: 10.1016/j.cbpc.2008.06.006. Epub 2008 Jun 22. Comp Biochem Physiol C Toxicol Pharmacol. 2008. PMID: 18621148 Free PMC article.
-
Japanese medaka (Oryzias latipes): developmental model for the study of alcohol teratology.Birth Defects Res B Dev Reprod Toxicol. 2006 Feb;77(1):29-39. doi: 10.1002/bdrb.20072. Birth Defects Res B Dev Reprod Toxicol. 2006. PMID: 16496295
-
DNA methyltransferase expressions in Japanese rice fish (Oryzias latipes) embryogenesis is developmentally regulated and modulated by ethanol and 5-azacytidine.Comp Biochem Physiol C Toxicol Pharmacol. 2015 Oct-Nov;176-177:1-9. doi: 10.1016/j.cbpc.2015.07.002. Epub 2015 Jul 13. Comp Biochem Physiol C Toxicol Pharmacol. 2015. PMID: 26183885
-
Modulation of ethanol toxicity by Asian ginseng (Panax ginseng) in Japanese ricefish (Oryzias latipes) embryogenesis.Comp Biochem Physiol C Toxicol Pharmacol. 2013 Apr;157(3):287-97. doi: 10.1016/j.cbpc.2013.02.001. Epub 2013 Feb 9. Comp Biochem Physiol C Toxicol Pharmacol. 2013. PMID: 23402931
-
Developmental ethanol exposure impairs locomotor movement in Japanese medaka (Oryzias latipes) larvae targeting epigenome.Chemosphere. 2017 Nov;186:901-910. doi: 10.1016/j.chemosphere.2017.08.048. Epub 2017 Aug 10. Chemosphere. 2017. PMID: 28826137
Cited by
-
Disruption of circulation by ethanol promotes fetal alcohol spectrum disorder (FASD) in medaka (Oryzias latipes) embryogenesis.Comp Biochem Physiol C Toxicol Pharmacol. 2008 Sep;148(3):273-80. doi: 10.1016/j.cbpc.2008.06.006. Epub 2008 Jun 22. Comp Biochem Physiol C Toxicol Pharmacol. 2008. PMID: 18621148 Free PMC article.
-
Finfish and aquatic invertebrate pathology resources for now and the future.Comp Biochem Physiol C Toxicol Pharmacol. 2009 Mar;149(2):249-57. doi: 10.1016/j.cbpc.2008.10.002. Epub 2008 Oct 9. Comp Biochem Physiol C Toxicol Pharmacol. 2009. PMID: 18948226 Free PMC article. Review.
-
Graphene-Based Nanomaterials Toxicity in Fish.Rev Environ Contam Toxicol. 2019;247:1-58. doi: 10.1007/398_2018_15. Rev Environ Contam Toxicol. 2019. PMID: 30413975 Free PMC article. Review.
-
Ethanol disrupts chondrification of the neurocranial cartilages in medaka embryos without affecting aldehyde dehydrogenase 1A2 (Aldh1A2) promoter methylation.Comp Biochem Physiol C Toxicol Pharmacol. 2009 Nov;150(4):495-502. doi: 10.1016/j.cbpc.2009.07.007. Epub 2009 Aug 3. Comp Biochem Physiol C Toxicol Pharmacol. 2009. PMID: 19651241 Free PMC article.
-
Ethanol-induced attenuation of oxidative stress is unable to alter mRNA expression pattern of catalase, glutathione reductase, glutathione-S-transferase (GST1A), and superoxide dismutase (SOD3) enzymes in Japanese rice fish (Oryzias latipes) embryogenesis.Comp Biochem Physiol C Toxicol Pharmacol. 2011 Jan;153(1):159-67. doi: 10.1016/j.cbpc.2010.10.002. Epub 2010 Oct 18. Comp Biochem Physiol C Toxicol Pharmacol. 2011. PMID: 20965276 Free PMC article.
References
-
- Abel EL, Hannigan JH. Maternal risk factors in fetal alcohol syndrome: provocative and permissive influences. Neurotoxicol Teratol. 1985;17:445–462. - PubMed
-
- Arenzana FJ, Carvan MJ, Aijon J, Sanchez-Gonzalez R, Arevalo R, Porteros A. Teratogenic effects of ethanol exposure on zebrafish visual system development. Neurotoxicol Teratol. 2006;28:342–328. - PubMed
-
- Bellusci S, Furuta Y, Rush MG, Henderson R, Winner G, Hogan BL. Involvement of sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development. 1997;124:53–63. - PubMed
-
- Bilotta J, Barnett JA, Hancock L, Saszik S. Ethanol exposure alters zebrafish development: A novel model of fetal alcohol syndrome. Neurotox Terato. 2004;26:737–743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

