The ePHD protein SPBP interacts with TopBP1 and together they co-operate to stimulate Ets1-mediated transcription
- PMID: 17913746
- PMCID: PMC2095823
- DOI: 10.1093/nar/gkm739
The ePHD protein SPBP interacts with TopBP1 and together they co-operate to stimulate Ets1-mediated transcription
Abstract
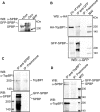
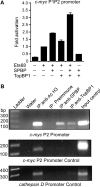
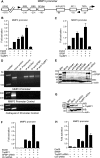
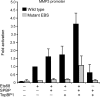
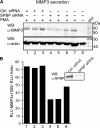
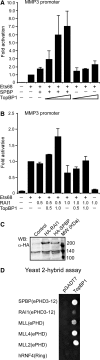
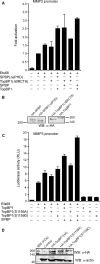
SPBP (Stromelysin-1 PDGF responsive element binding protein) is a ubiquitously expressed 220 kDa nuclear protein shown to enhance or repress the transcriptional activity of various transcription factors. A yeast two-hybrid screen, with the extended plant homeodomain (ePHD) of SPBP as bait, identified TopBP1 (topoisomerase II beta-binding protein 1) as a candidate interaction partner of SPBP. TopBP1 has eight BRCA1 carboxy-terminal (BRCT) domains and is involved in DNA replication, DNA damage responses and in the regulation of gene expression. The interaction between SPBP and TopBP1 was confirmed in vitro and in vivo, and was found to be mediated by the ePHD domain of SPBP and the BRCT6 domain of TopBP1. Both SPBP and TopBP1 enhanced the transcriptional activity of Ets1 on the c-myc P1P2- and matrix metalloproteinase-3 (MMP3) promoters. Together they displayed a more than additive effect. Both proteins were associated with these promoters. The involvement of TopBP1 was dependent on the serine 1159 phosphorylation site, known to be important for transcriptional activation. Depletion of endogenous SPBP by siRNA treatment reduced MMP3 secretion by 50% in phorbol ester-stimulated human fibroblasts. Taken together, our results show that TopBP1 and SPBP interact physically and functionally to co-operate as co-activators of Ets1.
Figures

Similar articles
-
Identification of two independent nucleosome-binding domains in the transcriptional co-activator SPBP.Biochem J. 2012 Feb 15;442(1):65-75. doi: 10.1042/BJ20111230. Biochem J. 2012. PMID: 22081970
-
A Functional interaction between the human papillomavirus 16 transcription/replication factor E2 and the DNA damage response protein TopBP1.J Biol Chem. 2002 Jun 21;277(25):22297-303. doi: 10.1074/jbc.M202163200. Epub 2002 Apr 4. J Biol Chem. 2002. PMID: 11934899
-
Characterization of the interaction between the human DNA topoisomerase IIbeta-binding protein 1 (TopBP1) and the cell division cycle 45 (Cdc45) protein.Biochem J. 2008 Jan 1;409(1):169-77. doi: 10.1042/BJ20070872. Biochem J. 2008. PMID: 17887956
-
The DNA topoisomerase IIbeta binding protein 1 (TopBP1) interacts with poly (ADP-ribose) polymerase (PARP-1).J Cell Biochem. 2007 Sep 1;102(1):171-82. doi: 10.1002/jcb.21292. J Cell Biochem. 2007. PMID: 17340632
-
TopBP1: A BRCT-scaffold protein functioning in multiple cellular pathways.DNA Repair (Amst). 2014 Oct;22:165-74. doi: 10.1016/j.dnarep.2014.06.004. Epub 2014 Jul 30. DNA Repair (Amst). 2014. PMID: 25087188 Review.
Cited by
-
p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription.J Biol Chem. 2010 Jul 16;285(29):22576-91. doi: 10.1074/jbc.M110.118976. Epub 2010 May 7. J Biol Chem. 2010. PMID: 20452972 Free PMC article.
-
TCF20 dysfunction leads to cortical neurogenesis defects and autistic-like behaviors in mice.EMBO Rep. 2020 Aug 5;21(8):e49239. doi: 10.15252/embr.201949239. Epub 2020 Jun 8. EMBO Rep. 2020. PMID: 32510763 Free PMC article.
-
SPBP is a sulforaphane induced transcriptional coactivator of NRF2 regulating expression of the autophagy receptor p62/SQSTM1.PLoS One. 2014 Jan 9;9(1):e85262. doi: 10.1371/journal.pone.0085262. eCollection 2014. PLoS One. 2014. PMID: 24416372 Free PMC article.
-
Transforming growth factor-β-inducible early response gene 1 is a novel substrate for atypical protein kinase Cs.Cell Mol Life Sci. 2011 Jun;68(11):1953-68. doi: 10.1007/s00018-010-0541-1. Epub 2010 Oct 17. Cell Mol Life Sci. 2011. PMID: 20953893 Free PMC article.
-
Rai1 haploinsufficiency causes reduced Bdnf expression resulting in hyperphagia, obesity and altered fat distribution in mice and humans with no evidence of metabolic syndrome.Hum Mol Genet. 2010 Oct 15;19(20):4026-42. doi: 10.1093/hmg/ddq317. Epub 2010 Jul 27. Hum Mol Genet. 2010. PMID: 20663924 Free PMC article.
References
-
- Rekdal C, Sjøttem E, Johansen T. The nuclear factor SPBP contains different functional domains and stimulates the activity of various transcriptional activators. J. Biol. Chem. 2000;275:40288–40300. - PubMed
-
- Lyngsø C, Bouteiller G, Damgaard CK, Ryom D, Sanchez-Munoz S, Norby PL, Bonven BJ, Jørgensen P. Interaction between the transcription factor SPBP and the positive cofactor RNF4. An interplay between protein binding zinc fingers. J. Biol. Chem. 2000;275:26144–26149. - PubMed
-
- Kirstein M, Sanz L, Quinones S, Moscat J, Diaz-Meco MT, Saus J. Cross-talk between different enhancer elements during mitogenic induction of the human stromelysin-1 gene. J. Biol. Chem. 1996;271:18231–18236. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

