Human diseases of telomerase dysfunction: insights into tissue aging
- PMID: 17913752
- PMCID: PMC2190725
- DOI: 10.1093/nar/gkm644
Human diseases of telomerase dysfunction: insights into tissue aging
Abstract
There are at least three human diseases that are associated with germ-line mutations of the genes encoding the two essential components of telomerase, TERT and TERC. Heterozygous mutations of these genes have been described for patients with dyskeratosis congenita, bone marrow failure and idiopathic pulmonary fibrosis. In this review, we will detail the clinical similarities and difference of these diseases and review the molecular phenotypes observed. The spectrum of mutations in TERT and TERC varies for these diseases and may in part explain the clinical differences observed. Environmental insults and genetic modifiers that accelerate telomere shortening and increase cell turnover may exaggerate the effects of telomerase haploinsufficiency, contributing to the variability of age of onset as well as tissue-specific organ pathology. A central still unanswered question is whether telomerase dysfunction and short telomeres are a much more prominent factor than previously suspected in other adult-onset, age-related diseases. Understanding the biological effects of these mutations may ultimately lead to novel treatments for these patients.
Figures
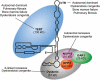


Similar articles
-
Recent progress in dyskeratosis congenita.Int J Hematol. 2010 Oct;92(3):419-24. doi: 10.1007/s12185-010-0695-5. Epub 2010 Oct 1. Int J Hematol. 2010. PMID: 20882440 Review.
-
Telomeres and marrow failure.Hematology Am Soc Hematol Educ Program. 2009:338-43. doi: 10.1182/asheducation-2009.1.338. Hematology Am Soc Hematol Educ Program. 2009. PMID: 20008219 Review.
-
Dyskeratosis congenita -- a disease of dysfunctional telomere maintenance.Curr Mol Med. 2005 Mar;5(2):159-70. doi: 10.2174/1566524053586581. Curr Mol Med. 2005. PMID: 15974869 Review.
-
Functional characterization of novel telomerase RNA (TERC) mutations in patients with diverse clinical and pathological presentations.Haematologica. 2007 Aug;92(8):1013-20. doi: 10.3324/haematol.11407. Epub 2007 Jul 20. Haematologica. 2007. PMID: 17640862 Free PMC article.
-
Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita.Blood. 2007 Sep 1;110(5):1439-47. doi: 10.1182/blood-2007-02-075598. Epub 2007 Apr 27. Blood. 2007. PMID: 17468339 Free PMC article. Clinical Trial.
Cited by
-
Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither?Epidemiol Rev. 2013;35(1):112-31. doi: 10.1093/epirev/mxs008. Epub 2013 Jan 9. Epidemiol Rev. 2013. PMID: 23302541 Free PMC article. Review.
-
Genomics, Telomere Length, Epigenetics, and Metabolomics in the Nurses' Health Studies.Am J Public Health. 2016 Sep;106(9):1663-8. doi: 10.2105/AJPH.2016.303344. Epub 2016 Jul 26. Am J Public Health. 2016. PMID: 27459442 Free PMC article. Review.
-
Dual regulation of TERT activity through transcription and splicing by DeltaNP63alpha.Aging (Albany NY). 2008 Dec 9;1(1):58-67. doi: 10.18632/aging.100003. Aging (Albany NY). 2008. PMID: 20157588 Free PMC article.
-
Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations.PLoS One. 2010 May 19;5(5):e10680. doi: 10.1371/journal.pone.0010680. PLoS One. 2010. PMID: 20502709 Free PMC article.
-
Telomeres and telomerase: three decades of progress.Nat Rev Genet. 2019 May;20(5):299-309. doi: 10.1038/s41576-019-0099-1. Nat Rev Genet. 2019. PMID: 30760854 Review.
References
-
- Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, et al. The RNA component of human telomerase. Science. 1995;269:1236–1241. - PubMed
-
- Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. - PubMed
-
- Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. - PubMed
-
- Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 1996;18:173–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

