Contribution of endocytic motifs in the cytoplasmic tail of herpes simplex virus type 1 glycoprotein B to virus replication and cell-cell fusion
- PMID: 17913800
- PMCID: PMC2168835
- DOI: 10.1128/JVI.01231-07
Contribution of endocytic motifs in the cytoplasmic tail of herpes simplex virus type 1 glycoprotein B to virus replication and cell-cell fusion
Abstract
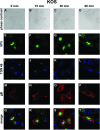
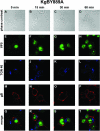
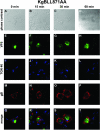
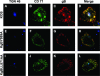
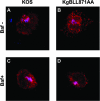
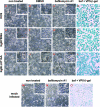
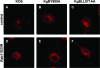
The use of endocytic pathways by viral glycoproteins is thought to play various functions during viral infection. We previously showed in transfection assays that herpes simplex virus type 1 (HSV-1) glycoprotein B (gB) is transported from the cell surface back to the trans-Golgi network (TGN) and that two motifs of gB cytoplasmic tail, YTQV and LL, function distinctly in this process. To investigate the role of each of these gB trafficking signals in HSV-1 infection, we constructed recombinant viruses in which each motif was rendered nonfunctional by alanine mutagenesis. In infected cells, wild-type gB was internalized from the cell surface and concentrated in the TGN. Disruption of YTQV abolished internalization of gB during infection, whereas disruption of LL induced accumulation of internalized gB in early recycling endosomes and impaired its return to the TGN. The growth of both recombinants was moderately diminished. Moreover, the fusion phenotype of cells infected with the gB recombinants differed from that of cells infected with the wild-type virus. Cells infected with the YTQV-mutated virus displayed reduced cell-cell fusion, whereas giant syncytia were observed in cells infected with the LL-mutated virus. Furthermore, blocking gB internalization or impairing gB recycling to the cell surface, using drugs or a transdominant negative form of Rab11, significantly reduced cell-cell fusion. These results favor a role for endocytosis in virus replication and suggest that gB intracellular trafficking is involved in the regulation of cell-cell fusion.
Figures
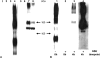





References
-
- Alconada, A., U. Bauer, L. Baudoux, J. Piette, and B. Hoflack. 1998. Intracellular transport of the glycoproteins gE and gI of the varicella-zoster virus: gE accelerates the maturation of gI and determines its accumulation in the trans-Golgi network. J. Biol. Chem. 273:13430-13436. - PubMed
-
- Avitabile, E., G. Lombardi, T. Gianni, M. Capri, and G. Campadelli-Fiume. 2004. Coexpression of UL20p and gK inhibits cell-cell fusion mediated by herpes simplex virus glycoproteins gD, gH-gL, and wild-type gB or an endocytosis-defective gB mutant and downmodulates their cell surface expression. J. Virol. 78:8015-8025. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

