Human herpesvirus 6A (HHV-6A) and HHV-6B alter E2F1/Rb pathways and E2F1 localization and cause cell cycle arrest in infected T cells
- PMID: 17913805
- PMCID: PMC2168879
- DOI: 10.1128/JVI.01496-07
Human herpesvirus 6A (HHV-6A) and HHV-6B alter E2F1/Rb pathways and E2F1 localization and cause cell cycle arrest in infected T cells
Abstract
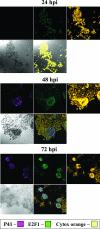
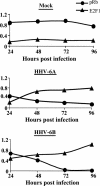
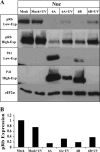
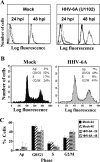
E2F transcription factors play pivotal roles in controlling the expression of genes involved in cell viability as well as genes involved in cell death. E2F1 is an important constituent of this protein family, which thus far contains eight members. The interaction of E2F1 with its major regulator, retinoblastoma protein (Rb), has been studied extensively in the past two decades, concentrating on the role of E2F1 in transcriptional regulation and the role of Rb in cell replication and cancer formation. Additionally, the effect of viral infections on E2F1/Rb interactions has been analyzed for different viruses, concentrating on cell division, which is essential for viral replication. In the present study, we monitored E2F1-Rb interactions during human herpesvirus 6A (HHV-6A) and HHV-6B infections of SupT1 T cells. The results have shown the following dramatic alterations in E2F1-Rb pathways compared to the pathways of parallel mock-infected control cultures. (i) The E2F1 levels were elevated during viral infections. (ii) The cellular localization of E2F1 was dramatically altered, and it was found to accumulate both in the cytoplasmic and nuclear fractions, as opposed to the strict nuclear localization seen in the mock-infected cells. (iii) Although E2F1 expression was elevated, two exemplary target genes, cyclin E and MCM5, were not upregulated. (iv) The Rb protein was dephosphorylated early postinfection, a trait that also occurred with UV-inactivated virus. (v) Infection was associated with significant reduction of E2F1/Rb complexing. (vi) HHV-6 infections were accompanied by cell cycle arrest. The altered E2F1-Rb interactions and functions might contribute to the observed cell cycle arrest.
Figures






Similar articles
-
Human herpesvirus 6 (HHV-6) alters E2F1/Rb pathways and utilizes the E2F1 transcription factor to express viral genes.Proc Natl Acad Sci U S A. 2014 Jan 7;111(1):451-6. doi: 10.1073/pnas.1308854110. Epub 2013 Dec 12. Proc Natl Acad Sci U S A. 2014. PMID: 24335704 Free PMC article.
-
Roseoloviruses manipulate host cell cycle.Curr Opin Virol. 2014 Dec;9:162-6. doi: 10.1016/j.coviro.2014.10.003. Epub 2014 Nov 10. Curr Opin Virol. 2014. PMID: 25462449 Review.
-
Human herpesvirus 6 suppresses T cell proliferation through induction of cell cycle arrest in infected cells in the G2/M phase.J Virol. 2011 Jul;85(13):6774-83. doi: 10.1128/JVI.02577-10. Epub 2011 Apr 27. J Virol. 2011. PMID: 21525341 Free PMC article.
-
RB·E2F1 complex mediates DNA damage responses through transcriptional regulation of ZBRK1.J Biol Chem. 2010 Oct 22;285(43):33134-33143. doi: 10.1074/jbc.M110.143461. Epub 2010 Aug 16. J Biol Chem. 2010. PMID: 20713352 Free PMC article.
-
Molecular biology of human herpesviruses 6A and 6B.Infect Agents Dis. 1993 Dec;2(6):343-60. Infect Agents Dis. 1993. PMID: 8012736 Review.
Cited by
-
Breaking Bad: How Viruses Subvert the Cell Cycle.Front Cell Infect Microbiol. 2018 Nov 19;8:396. doi: 10.3389/fcimb.2018.00396. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30510918 Free PMC article. Review.
-
Heterogenous circulating miRNA changes in ME/CFS converge on a unified cluster of target genes: A computational analysis.PLoS One. 2023 Dec 29;18(12):e0296060. doi: 10.1371/journal.pone.0296060. eCollection 2023. PLoS One. 2023. PMID: 38157384 Free PMC article.
-
Cloning human herpes virus 6A genome into bacterial artificial chromosomes and study of DNA replication intermediates.Proc Natl Acad Sci U S A. 2009 Nov 10;106(45):19138-43. doi: 10.1073/pnas.0908504106. Epub 2009 Oct 26. Proc Natl Acad Sci U S A. 2009. PMID: 19858479 Free PMC article.
-
The DR1 and DR6 first exons of human herpesvirus 6A are not required for virus replication in culture and are deleted in virus stocks that replicate well in T-cell lines.J Virol. 2010 Mar;84(6):2648-56. doi: 10.1128/JVI.01951-09. Epub 2010 Jan 6. J Virol. 2010. PMID: 20053742 Free PMC article.
-
Cell cycle perturbations induced by human herpesvirus 6 infection and their effect on virus replication.Arch Virol. 2014 Feb;159(2):365-70. doi: 10.1007/s00705-013-1826-0. Epub 2013 Sep 8. Arch Virol. 2014. PMID: 24013234 Free PMC article.
References
-
- Ablashi, A. H., Z. Berneman, et al. 1993. Human herpesvirus-6 strain groups: a nomenclature. Arch. Virol. 129:363-366. - PubMed
-
- Alvarez-Lafuente, R., V. de Las Heras, M. Garcia-Montojo, M. Bartolome, and R. Arroyo. 2007. Human herpesvirus-6 and multiple sclerosis: relapsing-remitting versus secondary progressive. Mult. Scler. 13:578-583. - PubMed
-
- Alvarez-Lafuente, R., M. Garcia-Montojo, V. De las Heras, M. Bartolome, and R. Arroyo. 2006. Clinical parameters and HHV-6 active replication in relapsing-remitting multiple sclerosis patients. J. Clin. Virol. 37(Suppl. 1):S24-S26. - PubMed
-
- Apostolova, M. D., I. A. Ivanova, C. Dagnino, S. J. D'Souza, and L. Dagnino. 2002. Active nuclear import and export pathways regulate E2F-5 subcellular localization. J. Biol. Chem. 277:34471-34479. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

