Human seminal plasma abrogates the capture and transmission of human immunodeficiency virus type 1 to CD4+ T cells mediated by DC-SIGN
- PMID: 17913809
- PMCID: PMC2168832
- DOI: 10.1128/JVI.01079-07
Human seminal plasma abrogates the capture and transmission of human immunodeficiency virus type 1 to CD4+ T cells mediated by DC-SIGN
Abstract
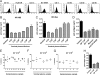
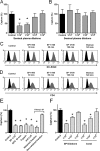
Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN) is expressed by dendritic cells (DCs) at mucosal surfaces and appears to play an important role in the dissemination of human immunodeficiency virus type 1 (HIV-1) infection. DC-SIGN binds HIV-1 gp120 and efficiently transmits the virus to T CD4(+) cells, which become the center of viral replication. Semen represents the main vector for HIV-1 dissemination worldwide. In the present study we show that human seminal plasma (SP), even when used at very high dilutions (1:10(4) to 1:10(5)), markedly inhibits the capture and transmission of HIV-1 to T CD4(+) cells mediated by both DCs and B-THP-1-DC-SIGN cells. In contrast, SP does not inhibit the capture of HIV-1 by DC-SIGN-negative target cells, such as the T-cell line SupT-1, monocytes, and activated peripheral blood mononuclear cells. The SP inhibitor has a high molecular mass (>100 kDa) and directly interacts with DC-SIGN-positive target cells but not with HIV-1. Moreover, the inhibitor binds to concanavalin A, suggesting that it contains high-mannose N-linked carbohydrates. Of note, using biotin-labeled SP we found that the binding of SP components to DCs was abrogated by mannan, while their interaction with B-THP-1 cells was almost completely dependent on the expression of DC-SIGN. Since epithelium integrity is often compromised after vaginal or anal intercourse, as well as in the presence of ulcerative-sexually transmitted diseases, our results support the notion that components of the SP might be able to access to the subepithelium, inhibiting the recognition of HIV-1 gp120 by DC-SIGN-positive DCs.
Figures





Similar articles
-
Role of the carbohydrate-binding sites of griffithsin in the prevention of DC-SIGN-mediated capture and transmission of HIV-1.PLoS One. 2013 May 31;8(5):e64132. doi: 10.1371/journal.pone.0064132. Print 2013. PLoS One. 2013. PMID: 23741304 Free PMC article.
-
Lentivirus-mediated RNA interference of DC-SIGN expression inhibits human immunodeficiency virus transmission from dendritic cells to T cells.J Virol. 2004 Oct;78(20):10848-55. doi: 10.1128/JVI.78.20.10848-10855.2004. J Virol. 2004. PMID: 15452205 Free PMC article.
-
Interaction of HIV-1 with dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin-expressing cells is influenced by gp120 envelope modifications associated with disease progression.FEBS J. 2006 Nov;273(21):4944-58. doi: 10.1111/j.1742-4658.2006.05491.x. Epub 2006 Sep 28. FEBS J. 2006. PMID: 17010165
-
DC-SIGN: a novel HIV receptor on DCs that mediates HIV-1 transmission.Curr Top Microbiol Immunol. 2003;276:31-54. doi: 10.1007/978-3-662-06508-2_2. Curr Top Microbiol Immunol. 2003. PMID: 12797442 Review.
-
DC-SIGN points the way to a novel mechanism for HIV-1 transmission.MedGenMed. 2003 May 23;5(2):2. MedGenMed. 2003. PMID: 14603101 Review.
Cited by
-
The High Content of Fructose in Human Semen Competitively Inhibits Broad and Potent Antivirals That Target High-Mannose Glycans.J Virol. 2020 Apr 16;94(9):e01749-19. doi: 10.1128/JVI.01749-19. Print 2020 Apr 16. J Virol. 2020. PMID: 32102878 Free PMC article.
-
Human Semen or Seminal Plasma Does Not Enhance HIV-1BaL Ex Vivo Infection of Human Colonic Explants.AIDS Res Hum Retroviruses. 2018 May;34(5):459-466. doi: 10.1089/AID.2017.0118. Epub 2018 Feb 21. AIDS Res Hum Retroviruses. 2018. PMID: 29343073 Free PMC article.
-
Circumcision as an Intervening Strategy against HIV Acquisition in the Male Genital Tract.Pathogens. 2021 Jun 25;10(7):806. doi: 10.3390/pathogens10070806. Pathogens. 2021. PMID: 34201976 Free PMC article. Review.
-
Mucosal transmission of human immunodeficiency virus.Curr HIV Res. 2012 Jan 1;10(1):3-8. doi: 10.2174/157016212799304689. Curr HIV Res. 2012. PMID: 22264040 Free PMC article. Review.
-
The Influence of Clusterin Glycosylation Variability on Selected Pathophysiological Processes in the Human Body.Oxid Med Cell Longev. 2022 Aug 28;2022:7657876. doi: 10.1155/2022/7657876. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36071866 Free PMC article. Review.
References
-
- Allen, R. D., and T. K. Roberts. 1986. The relationship between the immunosuppressive and cytotoxic effect of human seminal plasma. Am. J. Reprod. Immunol. Microbiol. 11:59-64. - PubMed
-
- Appelmelk, B. J., I. van Die, S. J. van Vliet, C. M. Vandenbroucke-Grauls, T. B. Geijtenbeek, and Y. van Kooyk. 2003. Carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 170:1635-1639. - PubMed
-
- Berlier, W., M. Cremel, H. Hamzeh, R. Lévy, F. Lucht, T. Bourlet, B. Pozzetto, and O. Delézayi. 2006. Seminal plasma promotes the attraction of Langerhans cells via the secretion of CCL20 by vaginal epithelial cells: involvement in the sexual transmission of HIV. Hum. Reprod. 21:1135-1142. - PubMed
-
- Bouhlal, H., N. Chomont, N. Haeffner-Cavaillon, M. D. Kazatchkine, L. Belec, and H. Hocini. 2002. Opsonization of HIV-1 by semen complement enhances infection of human epithelial cells. J. Immunol. 169:3301-3306. - PubMed
-
- Bouvet, J. P., G. Gresenguet, and L. Belec. 1997. Vaginal pH neutralization by semen as a cofactor of HIV transmission. Clin. Microbiol. Infect. 3:19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

