Chaperones activate hepadnavirus reverse transcriptase by transiently exposing a C-proximal region in the terminal protein domain that contributes to epsilon RNA binding
- PMID: 17913810
- PMCID: PMC2168843
- DOI: 10.1128/JVI.01196-07
Chaperones activate hepadnavirus reverse transcriptase by transiently exposing a C-proximal region in the terminal protein domain that contributes to epsilon RNA binding
Abstract
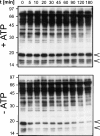
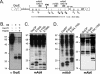
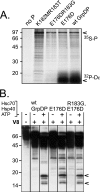
All hepatitis B viruses replicate by protein-primed reverse transcription, employing a specialized reverse transcriptase, P protein, that carries a unique terminal protein (TP) domain. To initiate reverse transcription, P protein must bind to a stem-loop, epsilon, on the pregenomic RNA template. TP then provides a Y residue for covalent attachment of the first nucleotide of an epsilon-templated DNA oligonucleotide (priming reaction) that serves to initiate full-length minus-strand DNA synthesis. epsilon binding requires the chaperone-dependent conversion of inactive P protein into an activated, metastable form designated P*. However, how P* differs structurally from P protein is not known. Here we used an in vitro reconstitution system for active duck hepatitis B virus P combined with limited proteolysis, site-specific antibodies, and defined P mutants to structurally compare nonactivated versus chaperone-activated versus primed P protein. The data show that Hsp70 action, under conditions identical to those required for functional activation, transiently exposes the C proximal TP region which is, probably directly, involved in epsilon RNA binding. Notably, after priming and epsilon RNA removal, a very similar new conformation appears stable without further chaperone activity; hence, the activation of P protein is triggered by energy-consuming chaperone action but may be completed by template RNA binding.
Figures

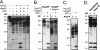

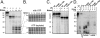

Similar articles
-
Chaperone activation of the hepadnaviral reverse transcriptase for template RNA binding is established by the Hsp70 and stimulated by the Hsp90 system.Nucleic Acids Res. 2007;35(18):6124-36. doi: 10.1093/nar/gkm628. Epub 2007 Sep 5. Nucleic Acids Res. 2007. PMID: 17804463 Free PMC article.
-
Heat shock protein 90-independent activation of truncated hepadnavirus reverse transcriptase.J Virol. 2003 Apr;77(8):4471-80. doi: 10.1128/jvi.77.8.4471-4480.2003. J Virol. 2003. PMID: 12663754 Free PMC article.
-
A Tyr residue in the reverse transcriptase domain can mimic the protein-priming Tyr residue in the terminal protein domain of a hepadnavirus P protein.J Virol. 2011 Aug;85(15):7742-53. doi: 10.1128/JVI.00482-11. Epub 2011 May 18. J Virol. 2011. PMID: 21593158 Free PMC article.
-
Hepatitis B viruses: reverse transcription a different way.Virus Res. 2008 Jun;134(1-2):235-49. doi: 10.1016/j.virusres.2007.12.024. Epub 2008 Mar 12. Virus Res. 2008. PMID: 18339439 Review.
-
Structure and function of the encapsidation signal of hepadnaviridae.J Viral Hepat. 1998 Nov;5(6):357-67. doi: 10.1046/j.1365-2893.1998.00124.x. J Viral Hepat. 1998. PMID: 9857345 Review.
Cited by
-
Hepatitis B Virus Nucleocapsid Assembly.J Mol Biol. 2025 Apr 30:169182. doi: 10.1016/j.jmb.2025.169182. Online ahead of print. J Mol Biol. 2025. PMID: 40316009 Review.
-
Identification and characterization of a novel bipartite nuclear localization signal in the hepatitis B virus polymerase.World J Gastroenterol. 2013 Nov 28;19(44):8000-10. doi: 10.3748/wjg.v19.i44.8000. World J Gastroenterol. 2013. PMID: 24307793 Free PMC article.
-
Functional and structural dynamics of hepadnavirus reverse transcriptase during protein-primed initiation of reverse transcription: effects of metal ions.J Virol. 2008 Jun;82(12):5703-14. doi: 10.1128/JVI.02760-07. Epub 2008 Apr 9. J Virol. 2008. PMID: 18400846 Free PMC article.
-
Protein-primed terminal transferase activity of hepatitis B virus polymerase.J Virol. 2013 Mar;87(5):2563-76. doi: 10.1128/JVI.02786-12. Epub 2012 Dec 19. J Virol. 2013. PMID: 23255788 Free PMC article.
-
Carbonyl J acid derivatives block protein priming of hepadnaviral P protein and DNA-dependent DNA synthesis activity of hepadnaviral nucleocapsids.J Virol. 2012 Sep;86(18):10079-92. doi: 10.1128/JVI.00816-12. Epub 2012 Jul 11. J Virol. 2012. PMID: 22787212 Free PMC article.
References
-
- Beck, J., and M. Nassal. 2003. Efficient Hsp90-independent in vitro activation by Hsc70 and Hsp40 of duck hepatitis B virus reverse transcriptase, an assumed Hsp90 client protein. J. Biol. Chem. 278:36128-36138. - PubMed
-
- Beck, J., and M. Nassal. 2004. In vitro reconstitution of epsilon-dependent duck hepatitis B virus replication initiation. Methods Mol. Med. 95:315-325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

