Human papillomavirus type 16 E7 oncoprotein associates with the centrosomal component gamma-tubulin
- PMID: 17913829
- PMCID: PMC2168839
- DOI: 10.1128/JVI.01669-07
Human papillomavirus type 16 E7 oncoprotein associates with the centrosomal component gamma-tubulin
Abstract
Expression of a high-risk human papillomavirus (HPV) E7 oncoprotein is sufficient to induce aberrant centrosome duplication in primary human cells. The resulting centrosome-associated mitotic abnormalities have been linked to the development of aneuploidy. HPV type 16 (HPV16) E7 induces supernumerary centrosomes through a mechanism that is at least in part independent of the inactivation of the retinoblastoma tumor suppressor pRb and is dependent on cyclin-dependent kinase 2 activity. Here, we show that HPV16 E7 can concentrate around mitotic spindle poles and that a small pool of HPV16 E7 is associated with centrosome fractions isolated by sucrose density gradient centrifugation. The targeting of HPV16 E7 to the centrosome, however, was not sufficient for centrosome overduplication. Nonetheless, we found that HPV16 E7 can associate with the centrosomal regulator gamma-tubulin and that the recruitment of gamma-tubulin to the centrosome is altered in HPV16 E7-expressing cells. Since the association of HPV16 E7 with gamma-tubulin is independent of pRb, p107, and p130, our results suggest that the association with gamma-tubulin contributes to the pRb/p107/p130-independent ability of HPV16 E7 to subvert centrosome homeostasis.
Figures
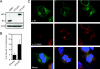


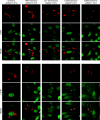

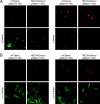
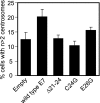

References
-
- Baker, S. J., S. Markowitz, E. R. Fearon, J. K. Willson, and B. Vogelstein. 1990. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science 249:912-915. - PubMed
-
- Brinkley, B. R., and T. M. Goepfert. 1998. Supernumerary centrosomes and cancer: Boveri's hypothesis resurrected. Cell Motil. Cytoskel. 41:281-288. - PubMed
-
- Crum, C. P., H. Ikenberg, R. M. Richart, and L. Gissman. 1984. Human papillomavirus type 16 and early cervical neoplasia. N. Engl. J. Med. 310:880-883. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

