Detection of histoplasma antigen by a quantitative enzyme immunoassay
- PMID: 17913863
- PMCID: PMC2168386
- DOI: 10.1128/CVI.00071-07
Detection of histoplasma antigen by a quantitative enzyme immunoassay
Abstract
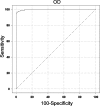
The second-generation Histoplasma antigen immunoassay is semiquantitative, expressing results as a comparison to a negative control, which requires repeat testing of the prior specimen with the current specimen to accurately determine a change in antigen. Reporting results in this manner often is confusing to the ordering physician and laboratory. Development of a quantitative assay could improve accuracy, reduce interassay variability, and eliminate the need to test the prior sample with the current sample in the same assay. Calibrators with known concentrations of Histoplasma antigen were used to quantitate antigen in specimens from patients with histoplasmosis and from controls. Samples from cases of disseminated histoplasmosis or other mycoses and controls were tested to evaluate the performance characteristics of the quantitative assay. Paired specimens were evaluated to determine if quantitation eliminated the need to test the current and prior specimens in the same assay to assess a change in antigen. The sensitivity in samples from patients with AIDS and disseminated histoplasmosis was 100% in urine and 92.3% in serum. Cross-reactions occurred in 70% of other endemic mycoses, but not in aspergillosis. Specificity was 99% in controls with community-acquired pneumonia, medical conditions in which histoplasmosis was excluded, or healthy subjects. A change in antigen level categorized as an increase, no change, or decrease based on antigen units determined in the same assay agreed closely with the category of change in nanograms/milliliter determined from testing current and prior specimens in different assays. Sensitivity, specificity, and interassay precision are excellent in the new third-generation quantitative Histoplasma antigen immunoassay.
Figures



References
-
- Azuma, I., F. Kanetsuna, Y. Tanaka, Y. Yamamura, and L. M. Carbonell. 1974. Chemical and immunological properties of galactomannans obtained from Histoplasma duboisii, Histoplasma capsulatum, Paracoccidioides brasiliensis and Blastomyces dermatitidis. Mycopathol. Mycol. Appl. 54:111-125. - PubMed
-
- Goldman, M., R. Zackin, C. J. Fichtenbaum, D. J. Skiest, S. L. Koletar, R. Hafner, L. J. Wheat, P. M. Nyangweso, C. T. Yiannoutsos, C. T. Schnizlein-Bick, S. Owens, and J. A. Aberg. 2004. Safety of discontinuation of maintenance therapy for disseminated histoplasmosis after immunologic response to antiretroviral therapy. Clin. Infect. Dis. 38:1485-1489. - PubMed
-
- Hecht, F. M., J. Wheat, A. H. Korzun, R. Hafner, K. J. Skahan, R. Larsen, M. T. Limjoco, M. Simpson, D. Schneider, M. C. Keefer, R. Clark, K. K. Lai, J. M. Jacobson, K. Squires, J. A. Bartlett, and W. Powderly. 1997. Itraconazole maintenance treatment for histoplasmosis in AIDS: a prospective, multicenter trial. J. Acquir. Immun. Defic. Syndr. Hum. Retrovir. 16:100-107. - PubMed
-
- Reference deleted.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

