Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output
- PMID: 17913900
- PMCID: PMC6672811
- DOI: 10.1523/JNEUROSCI.3134-07.2007
Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output
Abstract
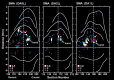
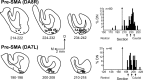
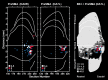
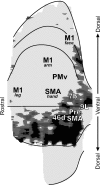
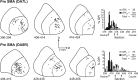
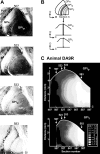
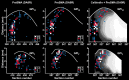
We used retrograde transneuronal transport of neurotropic viruses in Cebus monkeys to examine the organization of basal ganglia and cerebellar projections to two cortical areas on the medial wall of the hemisphere, the supplementary motor area (SMA) and the pre-SMA. We found that both of these cortical areas are the targets of disynaptic projections from the dentate nucleus of the cerebellum and from the internal segment of the globus pallidus (GPi). On average, the number of pallidal neurons that project to the SMA and pre-SMA is approximately three to four times greater than the number of dentate neurons that project to these cortical areas. GPi neurons that project to the pre-SMA are located in a rostral, "associative" territory of the nucleus, whereas GPi neurons that project to the SMA are located in a more caudal and ventral "sensorimotor" territory. Similarly, dentate neurons that project to the pre-SMA are located in a ventral, "nonmotor" domain of the nucleus, whereas dentate neurons that project to the SMA are located in a more dorsal, "motor" domain. The differential origin of subcortical projections to the SMA and pre-SMA suggests that these cortical areas are nodes in distinct neural systems. Although both systems are the target of outputs from the basal ganglia and the cerebellum, these two cortical areas seem to be dominated by basal ganglia input.
Figures





References
-
- Akkal D, Bioulac B, Audin J, Burbaud P. Comparison of neuronal activity in the rostral supplementary and cingulate motor areas during a task with cognitive and motor demands. Eur J Neurosci. 2002a;15:887–904. - PubMed
-
- Akkal D, Dum RP, Strick PL. Cerebellar and pallidal inputs to the pre-supplementary motor area (preSMA) Soc Neurosci Abstr. 2002b;28:462–14.
-
- Akkal D, Dum RP, Strick PL. Calbindin D-28K immunoreactivity reflects the topographic organization of motor and non-motor outputs channels in the globus pallidus. Soc Neurosci Abstr. 2003;29:601–4.
-
- Alexander GE, Crutcher MD. Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol. 1990;64:133–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
