Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome
- PMID: 17913902
- PMCID: PMC6672822
- DOI: 10.1523/JNEUROSCI.2624-07.2007
Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome
Abstract
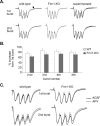
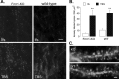
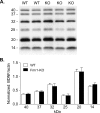
Mice lacking expression of the fragile X mental retardation 1 (Fmr1) gene have deficits in types of learning that are dependent on the hippocampus. Here, we report that long-term potentiation (LTP) elicited by threshold levels of theta burst afferent stimulation (TBS) is severely impaired in hippocampal field CA1 of young adult Fmr1 knock-out mice. The deficit was not associated with changes in postsynaptic responses to TBS, NMDA receptor activation, or levels of punctate glutamic acid decarboxylase-65/67 immunoreactivity. TBS-induced actin polymerization within dendritic spines was also normal. The LTP impairment was evident within 5 min of induction and, thus, may not be secondary to defects in activity-initiated protein synthesis. Protein levels for both brain-derived neurotrophic factor (BDNF), a neurotrophin that activates pathways involved in spine cytoskeletal reorganization, and its TrkB receptor were comparable between genotypes. BDNF infusion had no effect on baseline transmission or on postsynaptic responses to theta burst stimulation, but nonetheless fully restored LTP in slices from fragile X mice. These results indicate that the fragile X mutation produces a highly selective impairment to LTP, possibly at a step downstream of actin filament assembly, and suggest a means for overcoming this deficit. The possibility of a pharmacological therapy based on these results is discussed.
Figures



Similar articles
-
Modulation of dendritic spines and synaptic function by Rac1: a possible link to Fragile X syndrome pathology.Brain Res. 2011 Jul 5;1399:79-95. doi: 10.1016/j.brainres.2011.05.020. Epub 2011 May 17. Brain Res. 2011. PMID: 21645877 Free PMC article.
-
Physiological activation of synaptic Rac>PAK (p-21 activated kinase) signaling is defective in a mouse model of fragile X syndrome.J Neurosci. 2010 Aug 18;30(33):10977-84. doi: 10.1523/JNEUROSCI.1077-10.2010. J Neurosci. 2010. PMID: 20720104 Free PMC article.
-
Fragile X mental retardation protein regulates heterosynaptic plasticity in the hippocampus.Learn Mem. 2011 Mar 23;18(4):207-20. doi: 10.1101/lm.2043811. Print 2011. Learn Mem. 2011. PMID: 21430043 Free PMC article.
-
BDNF in fragile X syndrome.Neuropharmacology. 2014 Jan;76 Pt C:729-36. doi: 10.1016/j.neuropharm.2013.05.018. Epub 2013 May 29. Neuropharmacology. 2014. PMID: 23727436 Review.
-
BDNF mechanisms in late LTP formation: A synthesis and breakdown.Neuropharmacology. 2014 Jan;76 Pt C:664-76. doi: 10.1016/j.neuropharm.2013.06.024. Epub 2013 Jul 2. Neuropharmacology. 2014. PMID: 23831365 Review.
Cited by
-
Deficit in motor training-induced clustering, but not stabilization, of new dendritic spines in FMR1 knock-out mice.PLoS One. 2015 May 7;10(5):e0126572. doi: 10.1371/journal.pone.0126572. eCollection 2015. PLoS One. 2015. PMID: 25950728 Free PMC article.
-
Dendritic spine dysgenesis in autism related disorders.Neurosci Lett. 2015 Aug 5;601:30-40. doi: 10.1016/j.neulet.2015.01.011. Epub 2015 Jan 8. Neurosci Lett. 2015. PMID: 25578949 Free PMC article. Review.
-
Fmr1-KO mice failure to detect object novelty associates with a post-test decrease of structural and synaptic plasticity upstream of the hippocampus.Sci Rep. 2023 Jan 14;13(1):755. doi: 10.1038/s41598-023-27991-9. Sci Rep. 2023. PMID: 36641518 Free PMC article.
-
Altered structural and functional synaptic plasticity with motor skill learning in a mouse model of fragile X syndrome.J Neurosci. 2013 Dec 11;33(50):19715-23. doi: 10.1523/JNEUROSCI.2514-13.2013. J Neurosci. 2013. PMID: 24336735 Free PMC article.
-
Excitatory/Inhibitory Balance and Circuit Homeostasis in Autism Spectrum Disorders.Neuron. 2015 Aug 19;87(4):684-98. doi: 10.1016/j.neuron.2015.07.033. Neuron. 2015. PMID: 26291155 Free PMC article. Review.
References
-
- Arai A, Lynch G. Factors regulating the magnitude of long-term potentiation induced by theta pattern stimulation. Brain Res. 1992;598:173–184. - PubMed
-
- Biagini G, Avoli M, Marcinkiewicz J, Marcinkiewicz M. Brain-derived neurotrophic factor superinduction parallels anti-epileptic–neuroprotective treatment in the pilocarpine epilepsy model. J Neurochem. 2001;76:1814–1822. - PubMed
-
- Bramham C, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
