Inflammation activates the interferon signaling pathways in taste bud cells
- PMID: 17913904
- PMCID: PMC2096741
- DOI: 10.1523/JNEUROSCI.3102-07.2007
Inflammation activates the interferon signaling pathways in taste bud cells
Abstract
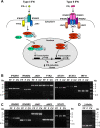
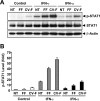
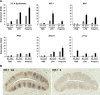
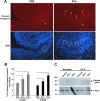
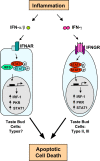
Patients with viral and bacterial infections or other inflammatory illnesses often experience taste dysfunctions. The agents responsible for these taste disorders are thought to be related to infection-induced inflammation, but the mechanisms are not known. As a first step in characterizing the possible role of inflammation in taste disorders, we report here evidence for the presence of interferon (IFN)-mediated signaling pathways in taste bud cells. IFN receptors, particularly the IFN-gamma receptor IFNGR1, are coexpressed with the taste cell-type markers neuronal cell adhesion molecule and alpha-gustducin, suggesting that both the taste receptor cells and synapse-forming cells in the taste bud can be stimulated by IFN. Incubation of taste bud-containing lingual epithelia with recombinant IFN-alpha and IFN-gamma triggered the IFN-mediated signaling cascades, resulting in the phosphorylation of the downstream STAT1 (signal transducer and activator of transcription protein 1) transcription factor. Intraperitoneal injection of lipopolysaccharide or polyinosinic:polycytidylic acid into mice, mimicking bacterial and viral infections, respectively, altered gene expression patterns in taste bud cells. Furthermore, the systemic administration of either IFN-alpha or IFN-gamma significantly increased the number of taste bud cells undergoing programmed cell death. These findings suggest that bacterial and viral infection-induced IFNs can act directly on taste bud cells, affecting their cellular function in taste transduction, and that IFN-induced apoptosis in taste buds may cause abnormal cell turnover and skew the representation of different taste bud cell types, leading to the development of taste disorders. To our knowledge, this is the first study providing direct evidence that inflammation can affect taste buds through cytokine signaling pathways.
Figures



Similar articles
-
Lipopolysaccharide-induced inflammation attenuates taste progenitor cell proliferation and shortens the life span of taste bud cells.BMC Neurosci. 2010 Jun 10;11:72. doi: 10.1186/1471-2202-11-72. BMC Neurosci. 2010. PMID: 20537148 Free PMC article.
-
Inflammation and taste disorders: mechanisms in taste buds.Ann N Y Acad Sci. 2009 Jul;1170:596-603. doi: 10.1111/j.1749-6632.2009.04480.x. Ann N Y Acad Sci. 2009. PMID: 19686199 Free PMC article.
-
Characterization of the expression pattern of adrenergic receptors in rat taste buds.Neuroscience. 2010 Sep 1;169(3):1421-37. doi: 10.1016/j.neuroscience.2010.05.021. Epub 2010 May 15. Neuroscience. 2010. PMID: 20478367 Free PMC article.
-
Signaling Pathways of Type I and Type III Interferons and Targeted Therapies in Systemic Lupus Erythematosus.Cells. 2019 Aug 23;8(9):963. doi: 10.3390/cells8090963. Cells. 2019. PMID: 31450787 Free PMC article. Review.
-
Taste bud homeostasis in health, disease, and aging.Chem Senses. 2014 Jan;39(1):3-16. doi: 10.1093/chemse/bjt059. Epub 2013 Nov 28. Chem Senses. 2014. PMID: 24287552 Free PMC article. Review.
Cited by
-
Cyclophosphamide has Long-Term Effects on Proliferation in Olfactory Epithelia.Chem Senses. 2020 Mar 25;45(2):97-109. doi: 10.1093/chemse/bjz075. Chem Senses. 2020. PMID: 31844905 Free PMC article.
-
Lipopolysaccharide-induced inflammation attenuates taste progenitor cell proliferation and shortens the life span of taste bud cells.BMC Neurosci. 2010 Jun 10;11:72. doi: 10.1186/1471-2202-11-72. BMC Neurosci. 2010. PMID: 20537148 Free PMC article.
-
Obesity and COVID-19: Oro-Naso-Sensory Perception.J Clin Med. 2020 Jul 8;9(7):2158. doi: 10.3390/jcm9072158. J Clin Med. 2020. PMID: 32650509 Free PMC article. Review.
-
Physiology of the tongue with emphasis on taste transduction.Physiol Rev. 2023 Apr 1;103(2):1193-1246. doi: 10.1152/physrev.00012.2022. Epub 2022 Nov 24. Physiol Rev. 2023. PMID: 36422992 Free PMC article. Review.
-
The T cells in peripheral taste tissue of healthy human adults: predominant memory T cells and Th-1 cells.Chem Senses. 2010 Jul;35(6):501-9. doi: 10.1093/chemse/bjq040. Epub 2010 May 9. Chem Senses. 2010. PMID: 20457570 Free PMC article.
References
-
- Abdollahi M, Radfar M. A review of drug-induced oral reactions. J Contemp Dent Pract. 2003;4:10–31. - PubMed
-
- Aubert A, Dantzer R. The taste of sickness: lipopolysaccharide-induced finickiness in rats. Physiol Behav. 2005;84:437–444. - PubMed
-
- Balachandran S, Roberts PC, Brown LE, Truong H, Pattnaik AK, Archer DR, Barber GN. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity. 2000;13:129–141. - PubMed
-
- Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. - PubMed
-
- Bartoshuk L, Desnoyers S, Hudson C, Marks L, O'Brien M. Tasting on localized areas. Ann NY Acad Sci. 1987;510:166–168.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
