Interactions between circadian neurons control temperature synchronization of Drosophila behavior
- PMID: 17913906
- PMCID: PMC6672815
- DOI: 10.1523/JNEUROSCI.2479-07.2007
Interactions between circadian neurons control temperature synchronization of Drosophila behavior
Abstract
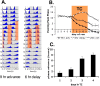
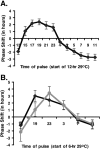
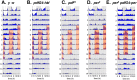
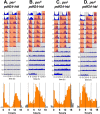
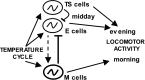
Most animals rely on circadian clocks to synchronize their physiology and behavior with the day/night cycle. Light and temperature are the major physical variables that can synchronize circadian rhythms. Although the effects of light on circadian behavior have been studied in detail in Drosophila, the neuronal mechanisms underlying temperature synchronization of circadian behavior have received less attention. Here, we show that temperature cycles synchronize and durably affect circadian behavior in Drosophila in the absence of light input. This synchronization depends on the well characterized and functionally coupled circadian neurons controlling the morning and evening activity under light/dark cycles: the M cells and E cells. However, circadian neurons distinct from the M and E cells are implicated in the control of rhythmic behavior specifically under temperature cycles. These additional neurons play a dual role: they promote evening activity and negatively regulate E cell function in the middle of the day. We also demonstrate that, although temperature synchronizes circadian behavior more slowly than light, this synchronization is considerably accelerated when the M cell oscillator is absent or genetically altered. Thus, whereas the E cells show great responsiveness to temperature input, the M cells and their robust self-sustained pacemaker act as a resistance to behavioral synchronization by temperature cycles. In conclusion, the behavioral responses to temperature input are determined by both the individual properties of specific groups of circadian neurons and their organization in a neural network.
Figures
Similar articles
-
Light activates output from evening neurons and inhibits output from morning neurons in the Drosophila circadian clock.PLoS Biol. 2007 Nov;5(11):e315. doi: 10.1371/journal.pbio.0050315. PLoS Biol. 2007. PMID: 18044989 Free PMC article.
-
A self-sustaining, light-entrainable circadian oscillator in the Drosophila brain.Curr Biol. 2003 Oct 14;13(20):1758-67. doi: 10.1016/j.cub.2003.09.030. Curr Biol. 2003. PMID: 14561400
-
miR-124 Regulates the Phase of Drosophila Circadian Locomotor Behavior.J Neurosci. 2016 Feb 10;36(6):2007-13. doi: 10.1523/JNEUROSCI.3286-15.2016. J Neurosci. 2016. PMID: 26865623 Free PMC article.
-
A plastic clock: how circadian rhythms respond to environmental cues in Drosophila.Mol Neurobiol. 2008 Oct;38(2):129-45. doi: 10.1007/s12035-008-8035-y. Epub 2008 Aug 27. Mol Neurobiol. 2008. PMID: 18751931 Review.
-
A fly's eye view of circadian entrainment.J Biol Rhythms. 2003 Jun;18(3):206-16. doi: 10.1177/0748730403018003003. J Biol Rhythms. 2003. PMID: 12828278 Review.
Cited by
-
Thermotaxis, circadian rhythms, and TRP channels in Drosophila.Temperature (Austin). 2015 Feb 11;2(2):227-43. doi: 10.1080/23328940.2015.1004972. eCollection 2015 Apr-Jun. Temperature (Austin). 2015. PMID: 27227026 Free PMC article. Review.
-
Pacemaker-neuron-dependent disturbance of the molecular clockwork by a Drosophila CLOCK mutant homologous to the mouse Clock mutation.Proc Natl Acad Sci U S A. 2016 Aug 16;113(33):E4904-13. doi: 10.1073/pnas.1523494113. Epub 2016 Aug 3. Proc Natl Acad Sci U S A. 2016. PMID: 27489346 Free PMC article.
-
Circadian Clock and Body Temperature.Adv Exp Med Biol. 2024;1461:177-188. doi: 10.1007/978-981-97-4584-5_12. Adv Exp Med Biol. 2024. PMID: 39289281 Review.
-
The Pyrexia transient receptor potential channel mediates circadian clock synchronization to low temperature cycles in Drosophila melanogaster.Proc Biol Sci. 2013 Oct 7;280(1768):20130959. doi: 10.1098/rspb.2013.0959. Print 2013 Oct 7. Proc Biol Sci. 2013. PMID: 23926145 Free PMC article.
-
Circadian Rhythms and Sleep in Drosophila melanogaster.Genetics. 2017 Apr;205(4):1373-1397. doi: 10.1534/genetics.115.185157. Genetics. 2017. PMID: 28360128 Free PMC article. Review.
References
-
- Allada R, White NE, So WV, Hall JC, Rosbash M. A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell. 1998;93:791–804. - PubMed
-
- Brown SA, Zumbrunn G, Fleury-Olela F, Preitner N, Schibler U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol. 2002;17:1574–1583. - PubMed
-
- Bruce VG. Biological clocks. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1960. Environmental entrainment of circadian rhythms; pp. 29–86.
-
- Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
