Estradiol improves cerebellar memory formation by activating estrogen receptor beta
- PMID: 17913916
- PMCID: PMC6672828
- DOI: 10.1523/JNEUROSCI.2588-07.2007
Estradiol improves cerebellar memory formation by activating estrogen receptor beta
Abstract
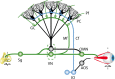
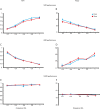
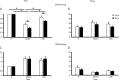
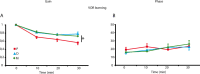
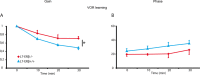
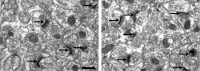
Learning motor skills is critical for motor abilities such as driving a car or playing piano. The speed at which we learn those skills is subject to many factors. Yet, it is not known to what extent gonadal hormones can affect the achievement of accurate movements in time and space. Here we demonstrate via different lines of evidence that estradiol promotes plasticity in the cerebellar cortex underlying motor learning. First, we show that estradiol enhances induction of long-term potentiation at the parallel fiber to Purkinje cell synapse, whereas it does not affect long-term depression; second, we show that estradiol activation of estrogen receptor beta receptors in Purkinje cells significantly improves gain-decrease adaptation of the vestibulo-ocular reflex, whereas it does not affect general eye movement performance; and third, we show that estradiol increases the density of parallel fiber to Purkinje cell synapses, whereas it does not affect the density of climbing fiber synapses. We conclude that estradiol can improve motor skills by potentiating cerebellar plasticity and synapse formation. These processes may be advantageous during periods of high estradiol levels of the estrous cycle or pregnancy.
Figures


References
-
- Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61.
-
- Andreescu CE, De Ruiter MM, De Zeeuw CI, De Jeu MT. Otolith deprivation induces optokinetic compensation. J Neurophysiol. 2005;94:3487–3496. - PubMed
-
- Barski JJ, Dethleffsen K, Meyer M. Cre recombinase expression in cerebellar Purkinje cells. Genesis. 2000;28:93–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
