Breadth of tuning and taste coding in mammalian taste buds
- PMID: 17913917
- PMCID: PMC3717408
- DOI: 10.1523/JNEUROSCI.1863-07.2007
Breadth of tuning and taste coding in mammalian taste buds
Erratum in
- J Neurosci. 2015 Jun 3;35(22):8683
Abstract
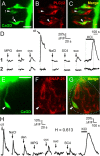
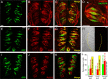
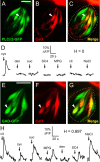
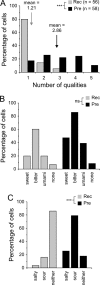
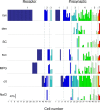
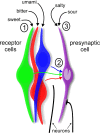
A longstanding question in taste research concerns taste coding and, in particular, how broadly are individual taste bud cells tuned to taste qualities (sweet, bitter, umami, salty, and sour). Taste bud cells express G-protein-coupled receptors for sweet, bitter, or umami tastes but not in combination. However, responses to multiple taste qualities have been recorded in individual taste cells. We and others have shown previously there are two classes of taste bud cells directly involved in gustatory signaling: "receptor" (type II) cells that detect and transduce sweet, bitter, and umami compounds, and "presynaptic" (type III) cells. We hypothesize that receptor cells transmit their signals to presynaptic cells. This communication between taste cells could represent a potential convergence of taste information in the taste bud, resulting in taste cells that would respond broadly to multiple taste stimuli. We tested this hypothesis using calcium imaging in a lingual slice preparation. Here, we show that receptor cells are indeed narrowly tuned: 82% responded to only one taste stimulus. In contrast, presynaptic cells are broadly tuned: 83% responded to two or more different taste qualities. Receptor cells responded to bitter, sweet, or umami stimuli but rarely to sour or salty stimuli. Presynaptic cells responded to all taste qualities, including sour and salty. These data further elaborate functional differences between receptor cells and presynaptic cells, provide strong evidence for communication within the taste bud, and resolve the paradox of broad taste cell tuning despite mutually exclusive receptor expression.
Figures

References
-
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
