The golgin GCC88 is required for efficient retrograde transport of cargo from the early endosomes to the trans-Golgi network
- PMID: 17914056
- PMCID: PMC2096601
- DOI: 10.1091/mbc.e07-06-0622
The golgin GCC88 is required for efficient retrograde transport of cargo from the early endosomes to the trans-Golgi network
Abstract
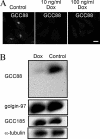
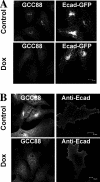
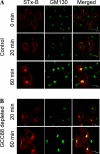
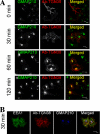
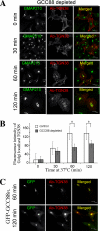
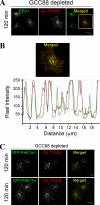
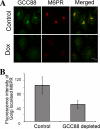
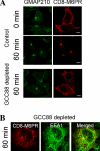
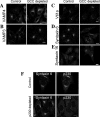
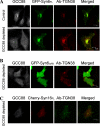
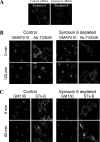
Retrograde transport pathways from early/recycling endosomes to the trans-Golgi network (TGN) are poorly defined. We have investigated the role of TGN golgins in retrograde trafficking. Of the four TGN golgins, p230/golgin-245, golgin-97, GCC185, and GCC88, we show that GCC88 defines a retrograde transport pathway from early endosomes to the TGN. Depletion of GCC88 in HeLa cells by interference RNA resulted in a block in plasma membrane-TGN recycling of two cargo proteins, TGN38 and a CD8 mannose-6-phosphate receptor cytoplasmic tail fusion protein. In GCC88-depleted cells, cargo recycling was blocked in the early endosome. Depletion of GCC88 dramatically altered the TGN localization of the t-SNARE syntaxin 6, a syntaxin required for endosome to TGN transport. Furthermore, the transport block in GCC88-depleted cells was rescued by syntaxin 6 overexpression. Internalized Shiga toxin was efficiently transported from endosomes to the Golgi of GCC88-depleted cells, indicating that Shiga toxin and TGN38 are internalized by distinct retrograde transport pathways. These findings have identified an essential role for GCC88 in the localization of TGN fusion machinery for transport from early endosomes to the TGN, and they have allowed the identification of a retrograde pathway which differentially selects TGN38 and mannose-6-phosphate receptor from Shiga toxin.
Figures

References
-
- Allan B. B., Moyer B. D., Balch W. E. Rab1 recruitment of p115 into a cis-SNARE complex: Programming budding COPII vesicles for fusion. Science. 2000;289:444–448. - PubMed
-
- Barr F. A. A novel Rab6-interacting domain defines a family of Golgi-targeted coiled-coil proteins. Curr. Biol. 1999;9:381–384. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

