Synaptotagmin C2A loop 2 mediates Ca2+-dependent SNARE interactions essential for Ca2+-triggered vesicle exocytosis
- PMID: 17914059
- PMCID: PMC2096586
- DOI: 10.1091/mbc.e07-04-0368
Synaptotagmin C2A loop 2 mediates Ca2+-dependent SNARE interactions essential for Ca2+-triggered vesicle exocytosis
Abstract
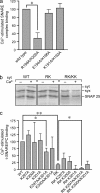
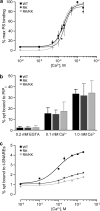
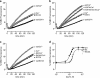
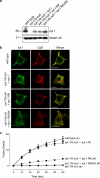
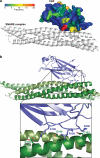
Synaptotagmins contain tandem C2 domains and function as Ca(2+) sensors for vesicle exocytosis but the mechanism for coupling Ca(2+) rises to membrane fusion remains undefined. Synaptotagmins bind SNAREs, essential components of the membrane fusion machinery, but the role of these interactions in Ca(2+)-triggered vesicle exocytosis has not been directly assessed. We identified sites on synaptotagmin-1 that mediate Ca(2+)-dependent SNAP25 binding by zero-length cross-linking. Mutation of these sites in C2A and C2B eliminated Ca(2+)-dependent synaptotagmin-1 binding to SNAREs without affecting Ca(2+)-dependent membrane binding. The mutants failed to confer Ca(2+) regulation on SNARE-dependent liposome fusion and failed to restore Ca(2+)-triggered vesicle exocytosis in synaptotagmin-deficient PC12 cells. The results provide direct evidence that Ca(2+)-dependent SNARE binding by synaptotagmin is essential for Ca(2+)-triggered vesicle exocytosis and that Ca(2+)-dependent membrane binding by itself is insufficient to trigger fusion. A structure-based model of the SNARE-binding surface of C2A provided a new view of how Ca(2+)-dependent SNARE and membrane binding occur simultaneously.
Figures
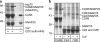


References
-
- Arac D., Murphy T., Rizo J. Facile detection of protein-protein interactions by one-dimensional NMR spectroscopy. Biochemistry. 2003;42:2774–2780. - PubMed
-
- Bai J., Wang C. T., Richards D. A., Jackson M. B., Chapman E. R. Fusion pore dynamics are regulated by synaptotagmin*t-SNARE interactions. Neuron. 2004;41:929–942. - PubMed
-
- Bennett M. K., Calakos N., Scheller R. H. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. - PubMed
-
- Bhalla A., Chicka M. C., Tucker W. C., Chapman E. R. Ca2+-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nat. Struct. Mol. Biol. 2006;13:323–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

