Differential regulation of Smac/DIABLO and Hsp-70 during brain maturation
- PMID: 17914183
- PMCID: PMC2755584
- DOI: 10.1007/s12017-007-8007-9
Differential regulation of Smac/DIABLO and Hsp-70 during brain maturation
Abstract
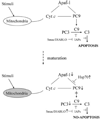
The heat shock protein (Hsp) system is a cell defense mechanism constitutively expressed at the basal state and essential for cell survival in response to damaging stimuli. Apoptosis is a physiological cell death program that preserves tissue homeostasis. We investigated the intrinsic pathway of apoptosis at various stages of brain maturation in CD-1 mice, triggered by two mitochondrial proapoptotic proteins, cytochrome c and Smac/DIABLO, and the pathway's regulation by Hsp-70. Smac/DIABLO and Hsp-70 proteins were upregulated 2-fold and 1.5-3-fold, respectively, after birth. In contrast, in the presence of cytochrome c/2'-deoxyadenosine 5'-triphosphate (dATP), caspase activity in mouse brain cell-free extracts increased 90-fold and 61-fold, at fetal and neonatal stages, whereas no activation was detected 15 days postnatally or at any subsequent times. These results indicate that the activation pattern of the intrinsic pathway of apoptosis undergoes a marked shift during postnatal maturation.
Figures



Similar articles
-
Mitochondrial fission leads to Smac/DIABLO release quenched by ARC.Apoptosis. 2010 Oct;15(10):1187-96. doi: 10.1007/s10495-010-0514-8. Apoptosis. 2010. PMID: 20552279
-
Interaction between XIAP and Smac/DIABLO in the mouse brain after transient focal cerebral ischemia.J Cereb Blood Flow Metab. 2003 Sep;23(9):1010-9. doi: 10.1097/01.WCB.0000080702.47016.FF. J Cereb Blood Flow Metab. 2003. PMID: 12973017
-
Heat shock pretreatment inhibited the release of Smac/DIABLO from mitochondria and apoptosis induced by hydrogen peroxide in cardiomyocytes and C2C12 myogenic cells.Cell Stress Chaperones. 2005 Autumn;10(3):252-62. doi: 10.1379/csc-124r.1. Cell Stress Chaperones. 2005. PMID: 16184770 Free PMC article.
-
X-linked inhibitor of apoptosis (XIAP) blocks Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis of prostate cancer cells in the presence of mitochondrial activation: sensitization by overexpression of second mitochondria-derived activator of caspase/direct IAP-binding protein with low pl (Smac/DIABLO).Mol Cancer Ther. 2002 Oct;1(12):1051-8. Mol Cancer Ther. 2002. PMID: 12481428
-
Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra.J Neuropathol Exp Neurol. 2003 Apr;62(4):329-39. doi: 10.1093/jnen/62.4.329. J Neuropathol Exp Neurol. 2003. PMID: 12722825 Review.
Cited by
-
MicroRNA-294 Regulates Apoptosis of the Porcine Cerebellum Caused by Selenium Deficiency via Targeting iNOS.Biol Trace Elem Res. 2021 Dec;199(12):4593-4603. doi: 10.1007/s12011-021-02583-8. Epub 2021 Jan 13. Biol Trace Elem Res. 2021. PMID: 33439455
-
The crosstalk between non-coding RNAs and oxidative stress in cancer progression.Genes Dis. 2024 Apr 10;12(3):101286. doi: 10.1016/j.gendis.2024.101286. eCollection 2025 May. Genes Dis. 2024. PMID: 40028033 Free PMC article. Review.
References
-
- Bartling B, Lewensohn R, Zhivotovsky B. Endogenously released Smac is insufficient to mediate cell death of human lung carcinoma in response to etoposide. Experimental Cell Research. 2004;298:83–95. - PubMed
-
- Beere HM. “The stress of dying”: The role of heat shock proteins in the regulation of apoptosis. Journal of Cell Science. 2004;117:2641–2651. - PubMed
-
- Beere HM, Wolf BB, Cain K, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nature Cell Biology. 2000;2:469–475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

