HCN4 provides a 'depolarization reserve' and is not required for heart rate acceleration in mice
- PMID: 17914461
- PMCID: PMC2063478
- DOI: 10.1038/sj.emboj.7601868
HCN4 provides a 'depolarization reserve' and is not required for heart rate acceleration in mice
Abstract
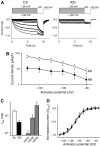
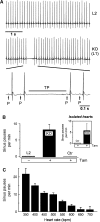
Cardiac pacemaking involves a variety of ion channels, but their relative importance is controversial and remains to be determined. Hyperpolarization-activated, cyclic nucleotide-gated (HCN) channels, which underlie the I(f) current of sinoatrial cells, are thought to be key players in cardiac automaticity. In addition, the increase in heart rate following beta-adrenergic stimulation has been attributed to the cAMP-mediated enhancement of HCN channel activity. We have now studied mice in which the predominant sinoatrial HCN channel isoform HCN4 was deleted in a temporally controlled manner. Here, we show that deletion of HCN4 in adult mice eliminates most of sinoatrial I(f) and results in a cardiac arrhythmia characterized by recurrent sinus pauses. However, the mutants show no impairment in heart rate acceleration during sympathetic stimulation. Our results reveal that unexpectedly the channel does not play a role for the increase of the heart rate; however, HCN4 is necessary for maintaining a stable cardiac rhythm, especially during the transition from stimulated to basal cardiac states.
Figures



References
-
- Biel M, Schneider A, Wahl C (2002) Cardiac HCN channels: structure, function, and modulation. Trends Cardiovasc Med 12: 206–212 - PubMed
-
- Brown HF, DiFrancesco D, Noble SJ (1979) How does adrenaline accelerate the heart? Nature 280: 235–236 - PubMed
-
- Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, Goodyear LJ, Kahn CR (1998) A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell 2: 559–569 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

