Anomeric reactivity-based one-pot synthesis of heparin-like oligosaccharides
- PMID: 17914818
- PMCID: PMC2525852
- DOI: 10.1021/ja073098r
Anomeric reactivity-based one-pot synthesis of heparin-like oligosaccharides
Abstract
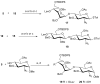
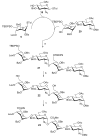
A highly efficient one-pot methodology is described for the synthesis of heparin and heparan sulfate oligosaccharides utilizing thioglycosides with well-defined reactivity as building blocks. L-Idopyranosyl and D-glucopyranosyl thioglycosides 5 and 10 were used as donors due to low reactivity of uronic acids as the glycosyl donors in the one-pot synthesis. The formation of uronic acids by a selective oxidation at C-6 was performed after assembly of the oligosaccharides. The efficiency of this programmable strategy with the flexibility for sulfate incorporation was demonstrated in the representative synthesis of disaccharides 17, 18, tetrasaccharide 23, and pentasaccharide 26.
Figures
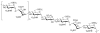

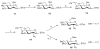
Similar articles
-
A modular strategy toward the synthesis of heparin-like oligosaccharides using monomeric building blocks in a sequential glycosylation strategy.J Am Chem Soc. 2005 Mar 23;127(11):3767-73. doi: 10.1021/ja045613g. J Am Chem Soc. 2005. PMID: 15771511
-
An efficient synthesis of L-idose and L-iduronic acid thioglycosides and their use for the synthesis of heparin oligosaccharides.Carbohydr Res. 2008 Mar 17;343(4):596-606. doi: 10.1016/j.carres.2007.12.015. Epub 2007 Dec 25. Carbohydr Res. 2008. PMID: 18237719
-
De novo synthesis of uronic acid building blocks for assembly of heparin oligosaccharides.Chemistry. 2007;13(16):4510-22. doi: 10.1002/chem.200700141. Chemistry. 2007. PMID: 17444537
-
Thioglycosides in sequential glycosylation strategies.Chem Soc Rev. 2005 Sep;34(9):769-82. doi: 10.1039/b417138c. Epub 2005 Jul 27. Chem Soc Rev. 2005. PMID: 16100617 Review.
-
Uronic acids in oligosaccharide and glycoconjugate synthesis.Top Curr Chem. 2011;301:253-89. doi: 10.1007/128_2010_111. Top Curr Chem. 2011. PMID: 21222193 Review.
Cited by
-
Can we produce heparin/heparan sulfate biomimetics using "mother-nature" as the gold standard?Molecules. 2015 Mar 5;20(3):4254-76. doi: 10.3390/molecules20034254. Molecules. 2015. PMID: 25751786 Free PMC article. Review.
-
Programmable one-pot synthesis of heparin pentasaccharides enabling access to regiodefined sulfate derivatives.Chem Sci. 2018 Jul 3;9(32):6685-6691. doi: 10.1039/c8sc01743c. eCollection 2018 Aug 28. Chem Sci. 2018. PMID: 30310602 Free PMC article.
-
Preactivation-based, one-pot combinatorial synthesis of heparin-like hexasaccharides for the analysis of heparin-protein interactions.Chemistry. 2010 Jul 26;16(28):8365-75. doi: 10.1002/chem.201000987. Chemistry. 2010. PMID: 20623566 Free PMC article.
-
Fluorous supported modular synthesis of heparan sulfate oligosaccharides.Org Lett. 2013 Jan 18;15(2):342-5. doi: 10.1021/ol303270v. Epub 2013 Jan 8. Org Lett. 2013. PMID: 23293947 Free PMC article.
-
Chemical O-Glycosylations: An Overview.ChemistryOpen. 2016 Aug 17;5(5):401-433. doi: 10.1002/open.201600043. eCollection 2016 Oct. ChemistryOpen. 2016. PMID: 27777833 Free PMC article. Review.
References
-
- Comper WD. Heparin and Related Polysaccharides. Vol. 1. Gordon and Breach; New York: 1981.
- Linhardt RJ, Toida T. In: Carbohydrates as Drugs. Witczak Z, Nieforth K, editors. Dekker; New York: 1998. pp. 277–341.
-
- Koshida S, Suda Y, Sobel M, Ormsby J, Kusumoto S. Bioorg Med Chem Lett. 1999;9:3127. - PubMed
- Petitou M, Duchaussoy P, Driguez PA, Jaurand G, Herault JP, Lormeau JC, van Boeckel CAA, Herbert JM. Angew Chem Int Ed. 1998;37:3009. - PubMed
- Petitou M, Herault JP, Bernat A, Driguez PA, Duchaussoy P, Lormeau JC, Herbert JM. Nature. 1999;398:417. - PubMed
-
- Jacquinet JC, Petitou M, Duchaussoy P, Lederman I, Choay J, Torri G, Sinay P. Carbohydr Res. 1984;130:221.
- Sinay P, Jacquinet JC, Petitou M, Duchaussoy P, Lederman I, Choay J, Torri G. Carbohydr Res. 1984;132:C5.
- Van Boeckel CAA, Beetz T, Vos JN, De Jong AJM, Van Aelst SF, Van den Bosch RH, Mertens JMR, Van der Vlugt FA. Carbohydr Res. 1985;4:293.
- Ichikawa Y, Monden R, Kuzuhara H. Carbohydr Res. 1988;172:37. - PubMed
-
-
Poletti L, Lay L. Eur J Org Chem. 2003:2999. and the references therein Noti C, Seeberger PH. Chem Biol. 2005;12:731. and the references therein Orgueira HA, Bartolozzi A, Schell P, Litiens REJN, Palmacci ER, Seeberger PH. Chem Eur J. 2003;9:140.Haller MF, Boons GJ. Eur J Org Chem. 2002:2033.de Paz JL, Ojeda R, Erichardt N, Martin-Lomas M. Eur J Org Chem. 2003;68:3308.Yu HY, Furukawa JI, Ikeda T, Wong CH. Org Lett. 2004;6:723.Lee JC, Lu XA, Kulkarni SS, Wen YS, Hung SC. J Am Chem Soc. 2004;126:476.Codee JDC, Stubba B, Schiattarella M, Overkleeft HS, van Boeckel CAA, van Boom JH, van der Marel GA. J Am Chem Soc. 2005;127:3767.Zhou Y, Lin F, Chen J, Yu B. Carbohydr Res. 2006;341:1619.Lu LD, Shie CR, Kulkarni SS, Pan GR, Lu XA, Hung SC. Org Lett. 2006;8:5995.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

