Force-induced unfolding of fibronectin in the extracellular matrix of living cells
- PMID: 17914904
- PMCID: PMC1994993
- DOI: 10.1371/journal.pbio.0050268
Force-induced unfolding of fibronectin in the extracellular matrix of living cells
Abstract
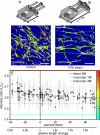
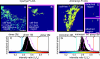
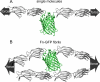
Whether mechanically unfolded fibronectin (Fn) is present within native extracellular matrix fibrils is controversial. Fn extensibility under the influence of cell traction forces has been proposed to originate either from the force-induced lengthening of an initially compact, folded quaternary structure as is found in solution (quaternary structure model, where the dimeric arms of Fn cross each other), or from the force-induced unfolding of type III modules (unfolding model). Clarification of this issue is central to our understanding of the structural arrangement of Fn within fibrils, the mechanism of fibrillogenesis, and whether cryptic sites, which are exposed by partial protein unfolding, can be exposed by cell-derived force. In order to differentiate between these two models, two fluorescence resonance energy transfer schemes to label plasma Fn were applied, with sensitivity to either compact-to-extended conformation (arm separation) without loss of secondary structure or compact-to-unfolded conformation. Fluorescence resonance energy transfer studies revealed that a significant fraction of fibrillar Fn within a three-dimensional human fibroblast matrix is partially unfolded. Complete relaxation of Fn fibrils led to a refolding of Fn. The compactly folded quaternary structure with crossed Fn arms, however, was never detected within extracellular matrix fibrils. We conclude that the resting state of Fn fibrils does not contain Fn molecules with crossed-over arms, and that the several-fold extensibility of Fn fibrils involves the unfolding of type III modules. This could imply that Fn might play a significant role in mechanotransduction processes.
Conflict of interest statement
Figures




References
-
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. - PubMed
-
- Vogel V. Mechanotransduction involving multimodular proteins: Converting force into biochemical signals. Annu Rev Biophys Biomol Struct. 2006;35:459–488. - PubMed
-
- Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. - PubMed
-
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. Nat Rev Mol Cell Biol. 2001;2:793–805. - PubMed
-
- Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005;24:389–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

