VEGF modulation of retinal pigment epithelium resistance
- PMID: 17915218
- PMCID: PMC2199266
- DOI: 10.1016/j.exer.2007.08.010
VEGF modulation of retinal pigment epithelium resistance
Abstract
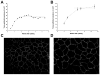
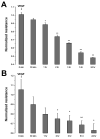
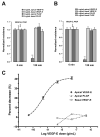
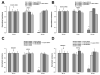
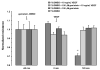
Fluid accumulation into the subretinal space and the development of macular edema is a common condition in age-related macular degeneration, diabetic retinopathy, and following ocular surgery, or injury. Vascular endothelial growth factor (VEGF) and other cytokines have been implicated in the disruption of retinal pigment epithelium (RPE) barrier function and a reduction in the regulated removal of subretinal fluid; however, the cellular and molecular events linking these agents to the disruption of barrier function have not been established. In the current study, cultures of ARPE-19 and primary porcine retinal pigment epithelium (RPE) cells were utilized to investigate the effects of the VEGF-induced modifications to the barrier properties of the RPE. The barrier function was determined by transepithelial resistance (TER) measurements and morphology of the RPE monolayers. In both ARPE-19 and primary porcine RPE cells the administration of VEGF produced a significant drop in TER, and this response was only observed following apical administration. Maximum reduction in TER was reached 5h post VEGF administration. These responses were concentration-dependent with an EC(50) of 502pg/mL in ARPE-19 cells and 251pg/mL in primary porcine cells. In both ARPE-19 and primary RPE cells, the response to VEGF was blocked by pretreatment with the relatively selective VEGF-R2 antagonists, SU5416 or ZM323881, or the protein tyrosine kinase inhibitor, genistein. Administration of the relatively selective VEGF-R2 agonist, VEGF-E, also reduced TER in a concentration-dependent manner (EC(50) of 474pg/mL), while VEGF-R1 agonist, placental growth factor (PlGF), did not significantly alter the TER. Immunolocalization studies demonstrated that confluent monolayers exhibited continuous cell-to-cell ZO-1 protein contacts and apical localization of the VEGF-R2 receptors. These data provide evidence that the VEGF-induced breakdown of RPE barrier function is mediated by the activation of apically-oriented VEGF-R2 receptors. Thus, VEGF-mediated increases in RPE permeability are initiated by a rise in intraocular levels of VEGF.
Figures
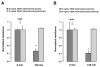


References
-
- Antcliff RJ, Marshall J. The pathogenesis of edema in diabetic maculopathy. Seminars in ophthalmology. 1999;14(4):223–232. - PubMed
-
- Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, Kroll J, Plaisance S, De Mol M, Bono F, Kliche S, Fellbrich G, Ballmer-Hofer K, Maglione D, Mayr-Beyrle U, Dewerchin M, Dombrowski S, Stanimirovic D, Van Hummelen P, Dehio C, Hicklin DJ, Persico G, Herbert JM, Shibuya M, Collen D, Conway EM, Carmeliet P. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9(7):936–943. - PubMed
-
- Barber AJ, Antonetti DA. Mapping the blood vessels with paracellular permeability in the retinas of diabetic rats. Invest Ophthalmol Vis Sci. 2003;44(12):5410–5416. - PubMed
-
- Becker PM, Waltenberger J, Yachechko R, Mirzapoiazova T, Sham JS, Lee CG, Elias JA, Verin AD. Neuropilin-1 regulates vascular endothelial growth factor-mediated endothelial permeability. Circulation research. 2005;96(12):1257–1265. - PubMed
-
- Blaauwgeers HG, Holtkamp GM, Rutten H, Witmer AN, Koolwijk P, Partanen TA, Alitalo K, Kroon ME, Kijlstra A, van Hinsbergh VW, Schlingemann RO. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol. 1999;155(2):421–428. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

