Immune evasion by acquisition of complement inhibitors: the mould Aspergillus binds both factor H and C4b binding protein
- PMID: 17915330
- PMCID: PMC5654503
- DOI: 10.1016/j.molimm.2007.08.011
Immune evasion by acquisition of complement inhibitors: the mould Aspergillus binds both factor H and C4b binding protein
Abstract
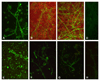
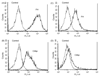
Pathogenic fungi represent a major threat particularly to immunocompromised hosts, leading to severe, and often lethal, systemic opportunistic infections. Although the impaired immune status of the host is clearly the most important factor leading to disease, virulence factors of the fungus also play a role. Factor H (FH) and its splice product FHL-1 represent the major fluid phase inhibitors of the alternative pathway of complement, whereas C4b-binding protein (C4bp) is the main fluid phase inhibitor of the classical and lectin pathways. Both proteins can bind to the surface of various human pathogens conveying resistance to complement destruction and thus contribute to their pathogenic potential. We have recently shown that Candida albicans evades complement by binding both Factor H and C4bp. Here we show that moulds such as Aspergillus spp. bind Factor H, the splicing variant FHL-1 and also C4bp. Immunofluorescence and flow cytometry studies show that the binding of Factor H and C4bp to Aspergillus spp. appears to be even stronger than to Candida spp. and that different, albeit possibly nearby, binding moieties mediate this surface attachment.
Figures




Similar articles
-
Enolase From Aspergillus fumigatus Is a Moonlighting Protein That Binds the Human Plasma Complement Proteins Factor H, FHL-1, C4BP, and Plasminogen.Front Immunol. 2019 Nov 22;10:2573. doi: 10.3389/fimmu.2019.02573. eCollection 2019. Front Immunol. 2019. PMID: 31824478 Free PMC article.
-
The hyphal and yeast forms of Candida albicans bind the complement regulator C4b-binding protein.Infect Immun. 2004 Nov;72(11):6633-41. doi: 10.1128/IAI.72.11.6633-6641.2004. Infect Immun. 2004. PMID: 15501796 Free PMC article.
-
The pH-regulated antigen 1 of Candida albicans binds the human complement inhibitor C4b-binding protein and mediates fungal complement evasion.J Biol Chem. 2011 Mar 11;286(10):8021-8029. doi: 10.1074/jbc.M110.130138. Epub 2011 Jan 6. J Biol Chem. 2011. PMID: 21212281 Free PMC article.
-
Diverse Functions of C4b-Binding Protein in Health and Disease.J Immunol. 2023 Nov 15;211(10):1443-1449. doi: 10.4049/jimmunol.2300333. J Immunol. 2023. PMID: 37931209 Free PMC article. Review.
-
Binding of complement regulatory proteins to group A Streptococcus.Vaccine. 2008 Dec 30;26 Suppl 8:I75-8. doi: 10.1016/j.vaccine.2008.11.054. Vaccine. 2008. PMID: 19388169 Review.
Cited by
-
Genome-wide transcriptional response of Silurana (Xenopus) tropicalis to infection with the deadly chytrid fungus.PLoS One. 2009 Aug 4;4(8):e6494. doi: 10.1371/journal.pone.0006494. PLoS One. 2009. PMID: 19701481 Free PMC article.
-
Functional characterization of LcpA, a surface-exposed protein of Leptospira spp. that binds the human complement regulator C4BP.Infect Immun. 2010 Jul;78(7):3207-16. doi: 10.1128/IAI.00279-10. Epub 2010 Apr 19. Infect Immun. 2010. PMID: 20404075 Free PMC article.
-
Complement Attack against Aspergillus and Corresponding Evasion Mechanisms.Interdiscip Perspect Infect Dis. 2012;2012:463794. doi: 10.1155/2012/463794. Epub 2012 Aug 9. Interdiscip Perspect Infect Dis. 2012. PMID: 22927844 Free PMC article.
-
Complement-Independent Modulation of Influenza A Virus Infection by Factor H.Front Immunol. 2020 Mar 25;11:355. doi: 10.3389/fimmu.2020.00355. eCollection 2020. Front Immunol. 2020. PMID: 32269562 Free PMC article.
-
Immune evasion of leptospira species by acquisition of human complement regulator C4BP.Infect Immun. 2009 Mar;77(3):1137-43. doi: 10.1128/IAI.01310-08. Epub 2008 Dec 29. Infect Immun. 2009. PMID: 19114549 Free PMC article.
References
-
- Berggard K, Johnsson E, Morfeldt E, Persson J, Stalhammar-Carlemalm M, Lindahl G. Binding of human C4BP to the hypervariable region of M protein: a molecular mechanism of phagocytosis resistance in Streptococcus pyogenes. Microbiol Mol. 2001a;42:539–551. - PubMed
-
- Berggard K, Lindahl G, Dahlback B, Blom AM. Bordetella pertussis binds to human C4b-binding protein (C4BP) at a site similar to that used by the natural ligand C4b. Eur J Immunol. 2001b;31:2771–2780. - PubMed
-
- Blom AM, Kask L, Dahlbäck B. Structural requirement for the complement regulatory activities of C4b-binding protein. J Biol Chem. 2001;276:27136–27144. - PubMed
-
- Blom AM, Villoutreix BO, Dahlback B. Complement inhibitor C4b-binding protein-friend or foe in the innate immune system? Mol Immunol. 2004;40:1333–1346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

