Validation of spermidine synthase as a drug target in African trypanosomes
- PMID: 17916066
- PMCID: PMC2427175
- DOI: 10.1042/BJ20071185
Validation of spermidine synthase as a drug target in African trypanosomes
Abstract
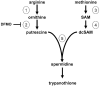
The trypanocidal activity of the ODC (ornithine decarboxylase) inhibitor DFMO (difluoromethylornithine) has validated polyamine biosynthesis as a target for chemotherapy. As DFMO is one of only two drugs used to treat patients with late-stage African trypanosomiasis, the requirement for additional drug targets is paramount. Here, we report the biochemical properties of TbSpSyn (Trypanosoma brucei spermidine synthase), the enzyme immediately downstream of ODC in this pathway. Recombinant TbSpSyn was purified and shown to catalyse the formation of spermidine from putrescine and dcSAM (decarboxylated S-adenosylmethionine). To determine the functional importance of TbSpSyn in BSF (bloodstream form) parasites, we used a tetracycline-inducible RNAi (RNA interference) system. Down-regulation of the corresponding mRNA correlated with a decrease in intracellular spermidine and cessation of growth. This phenotype could be complemented by expressing the SpSyn (spermidine synthase) gene from Leishmania major in cells undergoing RNAi, but could not be rescued by addition of spermidine to the medium due to the lack of a spermidine uptake capacity. These results therefore genetically validate TbSpSyn as a target for drug development and indicate that in the absence of a functional biosynthetic pathway, BSF T. brucei cannot scavenge sufficient spermidine from their environment to meet growth requirements.
Figures




References
-
- Barrett MP. The rise and fall of sleeping sickness. Lancet. 2006;367:1377–1378. - PubMed
-
- World Health Organization. Tropical Disease Research . 14th Programme Report of the UNDP/World Bank/WHO. Geneva: World Health Organization; 1999.
-
- Marton LJ, Pegg AE. Polyamines as targets for therapeutic intervention. Annu. Rev. Pharmacol. Toxicol. 1995;35:55–91. - PubMed
-
- Muller S, Coombs GH, Walter RD. Targeting polyamines of parasitic protozoa in chemotherapy. Trends Parasitol. 2001;17:242–249. - PubMed
-
- Bacchi CJ, Yarlett N. Polyamine metabolism as chemotherapeutic target in protozoan parasites. Mini Rev. Med. Chem. 2002;2:553–563. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

