LPS induces IL-10 production by human alveolar macrophages via MAPKinases- and Sp1-dependent mechanisms
- PMID: 17916230
- PMCID: PMC2080632
- DOI: 10.1186/1465-9921-8-71
LPS induces IL-10 production by human alveolar macrophages via MAPKinases- and Sp1-dependent mechanisms
Abstract
Background: IL-10 is a cytokine mainly produced by macrophages that plays key roles in tolerance to inhaled antigens and in lung homeostasis. Its regulation in alveolar macrophages (HAM), the resident lung phagocytes, remains however unknown.
Methods: The present study investigated the role of intracellular signalling and transcription factors controlling the production of IL-10 in LPS-activated HAM from normal nonsmoking volunteers.
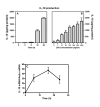
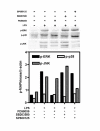
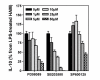
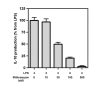
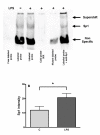
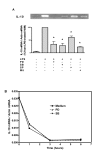
Results: LPS (1-1000 pg/ml) induced in vitro IL-10 production by HAM, both at mRNA and protein levels. LPS also activated the phosphorylation of ERK, p38 and JNK MAPkinases (immunoblots) and Sp-1 nuclear activity (EMSA). Selective inhibitors of MAPKinases (respectively PD98059, SB203580 and SP600125) and of Sp-1 signaling (mithramycin) decreased IL-10 expression in HAM. In addition, whilst not affecting IL-10 mRNA degradation, the three MAPKinase inhibitors completely abolished Sp-1 activation by LPS in HAM.
Conclusion: These results demonstrate for the first time that expression of IL-10 in lung macrophages stimulated by LPS depends on the concomitant activation of ERK, p38 and JNK MAPKinases, which control downstream signalling to Sp-1 transcription factor. This study further points to Sp-1 as a key signalling pathway for IL-10 expression in the lung.
Figures

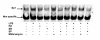
Similar articles
-
The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages.J Biol Chem. 2001 Apr 27;276(17):13664-74. doi: 10.1074/jbc.M011157200. Epub 2001 Jan 26. J Biol Chem. 2001. PMID: 11278848
-
c-Jun N-terminal kinase negatively regulates lipopolysaccharide-induced IL-12 production in human macrophages: role of mitogen-activated protein kinase in glutathione redox regulation of IL-12 production.J Immunol. 2003 Jul 15;171(2):628-35. doi: 10.4049/jimmunol.171.2.628. J Immunol. 2003. PMID: 12847227
-
Induction of VEGF gene transcription by IL-1 beta is mediated through stress-activated MAP kinases and Sp1 sites in cardiac myocytes.J Mol Cell Cardiol. 2000 Nov;32(11):1955-67. doi: 10.1006/jmcc.2000.1228. J Mol Cell Cardiol. 2000. PMID: 11040101
-
Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase.J Immunol. 1999 Dec 15;163(12):6403-12. J Immunol. 1999. PMID: 10586030
-
Mycobacteria-induced TNF-alpha and IL-10 formation by human macrophages is differentially regulated at the level of mitogen-activated protein kinase activity.J Immunol. 2001 Sep 15;167(6):3339-45. doi: 10.4049/jimmunol.167.6.3339. J Immunol. 2001. PMID: 11544323
Cited by
-
Toll-like receptor-mediated eosinophil-basophil differentiation: autocrine signalling by granulocyte-macrophage colony-stimulating factor in cord blood haematopoietic progenitors.Immunology. 2013 Jun;139(2):256-64. doi: 10.1111/imm.12078. Immunology. 2013. PMID: 23347362 Free PMC article.
-
Recombinant adiponectin protects the newborn rat lung from lipopolysaccharide-induced inflammatory injury.Physiol Rep. 2020 Sep;8(17):e14553. doi: 10.14814/phy2.14553. Physiol Rep. 2020. PMID: 32889775 Free PMC article.
-
Angiotensin AT2 receptor stimulation is anti-inflammatory in lipopolysaccharide-activated THP-1 macrophages via increased interleukin-10 production.Hypertens Res. 2015 Jan;38(1):21-9. doi: 10.1038/hr.2014.132. Epub 2014 Sep 11. Hypertens Res. 2015. PMID: 25209104 Free PMC article.
-
Cystatin C Deficiency Increases LPS-Induced Sepsis and NLRP3 Inflammasome Activation in Mice.Cells. 2021 Aug 12;10(8):2071. doi: 10.3390/cells10082071. Cells. 2021. PMID: 34440840 Free PMC article.
-
Effects of equine SALSA on neutrophil phagocytosis and macrophage cytokine production.PLoS One. 2022 Mar 14;17(3):e0264911. doi: 10.1371/journal.pone.0264911. eCollection 2022. PLoS One. 2022. PMID: 35286327 Free PMC article.
References
-
- Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

