Modulation of the cAMP signaling pathway after traumatic brain injury
- PMID: 17916353
- PMCID: PMC2141537
- DOI: 10.1016/j.expneurol.2007.08.011
Modulation of the cAMP signaling pathway after traumatic brain injury
Abstract
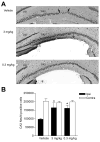
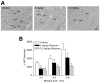
Traumatic brain injury (TBI) results in both focal and diffuse brain pathologies that are exacerbated by the inflammatory response and progress from hours to days after the initial injury. Using a clinically relevant model of TBI, the parasagittal fluid-percussion brain injury (FPI) model, we found injury-induced impairments in the cyclic AMP (cAMP) signaling pathway. Levels of cAMP were depressed in the ipsilateral parietal cortex and hippocampus, as well as activation of its downstream target, protein kinase A, from 15 min to 48 h after moderate FPI. To determine if preventing hydrolysis of cAMP by administration of a phosphodiesterase (PDE) IV inhibitor would improve outcome after TBI, we treated animals intraperitoneally with rolipram (0.3 or 3.0 mg/kg) 30 min prior to TBI, and then once per day for 3 days. Rolipram treatment restored cAMP to sham levels and significantly reduced cortical contusion volume and improved neuronal cell survival in the parietal cortex and CA3 region of the hippocampus. Traumatic axonal injury, characterized by beta-amyloid precursor protein deposits in the external capsule, was also significantly reduced in rolipram-treated animals. Furthermore, levels of the pro-inflammatory cytokines, interleukin-1beta (IL-1beta) and tumor necrosis factor-alpha (TNF-alpha), were significantly decreased with rolipram treatment. These results demonstrate that the cAMP-PKA signaling cascade is downregulated after TBI, and that treatment with a PDE IV inhibitor improves histopathological outcome and decreases inflammation after TBI.
Figures
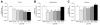






References
-
- Armstead WM. Role of impaired cAMP and calcium-sensitive K+ channel function in altered cerebral hemodynamics following brain injury. Brain Res. 1997;768:177–184. - PubMed
-
- Atkins CM, Chen S, Alonso OF, Dietrich WD, Hu BR. Activation of calcium/calmodulin-dependent protein kinases after traumatic brain injury. J Cereb Blood Flow Metab. 2006;26:1507–1518. - PubMed
-
- Bell MJ, Kochanek PM, Carcillo JA, Mi Z, Schiding JK, Wisniewski SR, Clark RS, Dixon CE, Marion DW, Jackson E. Interstitial adenosine, inosine, and hypoxanthine are increased after experimental traumatic brain injury in the rat. J Neurotrauma. 1998;15:163–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

