Cyclic AMP regulates bicarbonate secretion in cholangiocytes through release of ATP into bile
- PMID: 17916355
- PMCID: PMC2128713
- DOI: 10.1053/j.gastro.2007.08.020
Cyclic AMP regulates bicarbonate secretion in cholangiocytes through release of ATP into bile
Abstract
Background & aims: Bicarbonate secretion is a primary function of cholangiocytes. Either adenosine 3',5'-cyclic monophosphate (cAMP) or cytosolic Ca(2+) can mediate bicarbonate secretion, but these are thought to act through separate pathways. We examined the role of the inositol 1,4,5-trisphosphate receptor (InsP3R) in mediating bicarbonate secretion because this is the only intracellular Ca(2+) release channel in cholangiocytes.
Methods: Intrahepatic bile duct units (IBDUs) were microdissected from rat liver then luminal pH was examined by confocal microscopy during IBDU microperfusion. Cyclic AMP was increased using forskolin or secretin, and Ca(2+) was increased using acetylcholine (ACh) or adenosine triphosphate (ATP). Apyrase was used to hydrolyze extracellular ATP, and suramin was used to block apical P2Y ATP receptors. In selected experiments, IBDUs were pretreated with short interfering RNA (siRNA) to silence expression of specific InsP3R isoforms.
Results: Both cAMP and Ca(2+) agonists increased luminal pH. The effect of ACh on luminal pH was reduced by siRNA for basolateral (types I and II) but not apical (type III) InsP3R isoforms. The effect of forskolin on luminal pH was reduced by a cystic fibrosis transmembrane conductance regulator (CFTR) inhibitor and by siRNA for the type III InsP3R. Luminal apyrase or suramin blocked the effects of forskolin but not ACh on luminal pH.
Conclusions: Cyclic AMP-induced ductular bicarbonate secretion depends on an autocrine signaling pathway that involves CFTR, apical release of ATP, stimulation of apical nucleotide receptors, and then activation of apical, type III InsP3Rs. The primary role of CFTR in bile duct secretion may be to regulate secretion of ATP rather than to secrete chloride and/or bicarbonate.
Figures


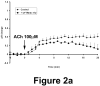

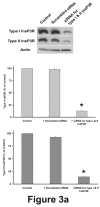




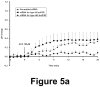













Comment in
-
Thinking outside the cell: the role of extracellular adenosine triphosphate in bile formation.Gastroenterology. 2007 Nov;133(5):1726-8. doi: 10.1053/j.gastro.2007.09.050. Gastroenterology. 2007. PMID: 17983816 No abstract available.
References
-
- Hirata K, Nathanson MH. Bile duct epithelia regulate biliary bicarbonate excretion in normal rat liver. Gastroenterology. 2001;121:396–406. - PubMed
-
- Boyer JL, Nathanson MH. Bile formation. In: Schiff ER, Sorrell MF, Maddrey WC, editors. Diseases of the liver. 1999. pp. 119–146.
-
- Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. - PubMed
-
- Kanno N, LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 2001;281:G612–G625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

