Heparin-mimetic sulfated peptides with modulated affinities for heparin-binding peptides and growth factors
- PMID: 17916399
- PMCID: PMC3100587
- DOI: 10.1016/j.peptides.2007.08.010
Heparin-mimetic sulfated peptides with modulated affinities for heparin-binding peptides and growth factors
Abstract
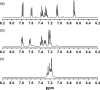
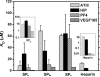
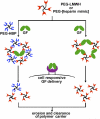
Heterogeneity in the composition and in the polydispersity of heparin has motivated the development of homogeneous heparin mimics, and peptides of appropriate sequence and chemical function have therefore recently emerged as potential replacements for heparin in selected applications. Here, we report the assessment of the binding affinities of multiple sulfated peptides (SPs) for a set of heparin-binding peptides (HBPs) and for vascular endothelial growth factor isoform 165 (VEGF165); these binding partners have application in the selective immobilization of proteins and in hydrogel formation through non-covalent interactions. Sulfated peptides were produced via solid-phase methods, and their affinity for the HBPs and VEGF165 was assessed via affinity liquid chromatography (ALC), surface plasmon resonance (SPR), and in selected cases, isothermal titration calorimetry (ITC). The shortest peptide, SP(a), showed the highest affinity binding of HBPs and VEGF165 in both ALC and SPR measurements, with slight exceptions. Of the investigated HBPs, a peptide based on the heparin-binding domain of human platelet factor 4 showed greatest binding affinities toward all of the SPs, consistent with its stronger binding to heparin. The affinity between SP(a) and PF4(ZIP) was indicated via SPR (K(D)=5.27 microM) and confirmed via ITC (K(D)=8.09 microM). The binding by SP(a) of both VEGF and HBPs suggests its use as a binding partner to multiple species, and the use of these interactions in assembly of materials. Given that the peptide sequences can be varied to control binding affinity and selectivity, opportunities are also suggested for the production of a wider array of matrices with selective binding and release properties useful for biomaterials applications.
Figures


Similar articles
-
Manipulation of hydrogel assembly and growth factor delivery via the use of peptide-polysaccharide interactions.J Control Release. 2006 Aug 28;114(2):130-42. doi: 10.1016/j.jconrel.2006.06.005. Epub 2006 Jun 10. J Control Release. 2006. PMID: 16890321 Free PMC article.
-
Characterization of heparin binding by a peptide from amyloid P component using capillary electrophoresis, surface plasmon resonance and isothermal titration calorimetry.Eur J Biochem. 2002 Jun;269(12):2860-7. doi: 10.1046/j.1432-1033.2002.02964.x. Eur J Biochem. 2002. PMID: 12071948
-
Heparin-von Willebrand factor binding as assessed by isothermal titration calorimetry and by affinity fractionation of heparins using synthetic peptides.Arch Biochem Biophys. 1993 Nov 1;306(2):528-33. doi: 10.1006/abbi.1993.1548. Arch Biochem Biophys. 1993. PMID: 8215459
-
Arginine functionalization of hydrogels for heparin binding--a supramolecular approach to developing a pro-angiogenic biomaterial.Biotechnol Bioeng. 2013 Jan;110(1):296-317. doi: 10.1002/bit.24598. Epub 2012 Jul 25. Biotechnol Bioeng. 2013. PMID: 22753043
-
Chemistry and biology of heparin mimetics that bind to fibroblast growth factors.Mini Rev Med Chem. 2007 Dec;7(12):1206-35. doi: 10.2174/138955707782795665. Mini Rev Med Chem. 2007. PMID: 18220975 Review.
Cited by
-
Bioactive scaffolds for engineering vascularized cardiac tissues.Macromol Biosci. 2010 Nov 10;10(11):1286-301. doi: 10.1002/mabi.201000202. Macromol Biosci. 2010. PMID: 20857391 Free PMC article.
-
Tissue engineering-based therapeutic strategies for vocal fold repair and regeneration.Biomaterials. 2016 Nov;108:91-110. doi: 10.1016/j.biomaterials.2016.08.054. Epub 2016 Sep 2. Biomaterials. 2016. PMID: 27619243 Free PMC article. Review.
-
Incorporation of Sulfated Hyaluronic Acid Macromers into Degradable Hydrogel Scaffolds for Sustained Molecule Delivery.Biomater Sci. 2014;2:693-702. doi: 10.1039/C3BM60227C. Biomater Sci. 2014. PMID: 24955239 Free PMC article.
-
Material surface conjugated with fibroblast growth factor-2 for pluripotent stem cell culture and differentiation.Regen Biomater. 2025 Jan 2;12:rbaf003. doi: 10.1093/rb/rbaf003. eCollection 2025. Regen Biomater. 2025. PMID: 39967781 Free PMC article.
-
PEG hydrogels for the controlled release of biomolecules in regenerative medicine.Pharm Res. 2009 Mar;26(3):631-43. doi: 10.1007/s11095-008-9801-2. Epub 2008 Dec 18. Pharm Res. 2009. PMID: 19089601 Free PMC article. Review.
References
-
- Baeg G, Lin X, Khare N, Baumgartner S, Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001;128:87–94. - PubMed
-
- Bagdy D, Barabas E, Gráf L, Petersen TE, Magnusson S. Hirudin. Methods Enzymol. 1976;45:669–78. - PubMed
-
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, et al. Functions of cell surface-heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–77. - PubMed
-
- Bollhagen R, Schmiedberger M, Barlos K, Grell E. A new reagent for the cleavage of fully protected peptides synthesized on 2-chlorotrityl chloride resin. J Chem Soc Chem Commun. 1994;22:2559–60.
-
- Butcher DJ, Kowalska MA, Li S, Luo Z, Shan S, Lu Z, et al. A natural motif approach to protein design: a synthetic leucine zipper peptide mimics the biological function of the platelet factor 4 protein. FEBS Lett. 1997;409:183–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

