The narcotic bowel syndrome: clinical features, pathophysiology, and management
- PMID: 17916540
- PMCID: PMC2074872
- DOI: 10.1016/j.cgh.2007.06.013
The narcotic bowel syndrome: clinical features, pathophysiology, and management
Abstract
Narcotic bowel syndrome (NBS) is a subset of opioid bowel dysfunction that is characterized by chronic or frequently recurring abdominal pain that worsens with continued or escalating dosages of narcotics. This syndrome is underrecognized and may be becoming more prevalent. In the United States this may be the result of increases in using narcotics for chronic nonmalignant painful disorders, and the development of maladaptive therapeutic interactions around its use. NBS can occur in patients with no prior gastrointestinal disorder who receive high dosages of narcotics after surgery or acute painful problems, and among patients with functional gastrointestinal disorders or other chronic gastrointestinal diseases who are managed by physicians who are unaware of the hyperalgesic effects of chronic opioids. The evidence for the enhanced pain perception is based on the following: (1) activation of excitatory antianalgesic pathways within a bimodal opioid regulation system, (2) descending facilitation of pain at the rostral ventral medulla and pain facilitation via dynorphin and cholecystokinin activation, and (3) glial cell activation that produces morphine tolerance and enhances opioid-induced pain. Treatment involves early recognition of the syndrome, an effective physician-patient relationship, graded withdrawal of the narcotic according to a specified withdrawal program, and the institution of medications to reduce withdrawal effects.
Figures

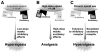


References
-
- Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg. 2001;182:11S–8S. - PubMed
-
- Mehendale SR, Yuan CS. Opioid-induced gastrointestinal dysfunction. Dig Dis. 2006;24:105–12. - PubMed
-
- Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs. 2003;63:649–71. - PubMed
-
- Sandgren JE, McPhee MS, Greenberger NJ. Narcotic bowel syndrome treated with Clonidine. Ann Intern Med. 1984;101:331–34. - PubMed
-
- Rogers M, Cerda JJ. Editorial: The narcotic bowel syndrome. J Clin Gastroenterol. 1989;11:132–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

