Assessment of hepatic fibrosis with magnetic resonance elastography
- PMID: 17916548
- PMCID: PMC2276978
- DOI: 10.1016/j.cgh.2007.06.012
Assessment of hepatic fibrosis with magnetic resonance elastography
Abstract
Background & aims: Accurate detection of hepatic fibrosis is crucial for assessing prognosis and candidacy for treatment in patients with chronic liver disease. Magnetic resonance (MR) elastography, a technique for quantitatively assessing the mechanical properties of soft tissues, has been shown previously to have potential for noninvasively detecting liver fibrosis. The goal of this work was to obtain preliminary estimates of the sensitivity and specificity of the technique in diagnosing liver fibrosis, and to assess its potential for identifying patients who potentially can avoid a biopsy procedure.
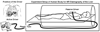
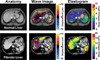
Methods: MR elastography was performed in 35 normal volunteers and 50 patients with chronic liver disease. MR imaging measurements of hepatic fat to water ratios were obtained to assess the potential for fat infiltration to affect stiffness-based detection of fibrosis.
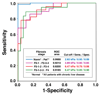
Results: Liver stiffness increased systematically with fibrosis stage. Receiver operating curve analysis showed that, with a shear stiffness cut-off value of 2.93 kilopascals, the predicted sensitivity and specificity for detecting all grades of liver fibrosis is 98% and 99%, respectively. Receiver operating curve analysis also provided evidence that MR elastography can discriminate between patients with moderate and severe fibrosis (grades 2-4) and those with mild fibrosis (sensitivity, 86%; specificity, 85%). Hepatic stiffness does not appear to be influenced by the degree of steatosis.
Conclusions: MR elastography is a safe, noninvasive technique with excellent diagnostic accuracy for assessing hepatic fibrosis. Based on the high negative predictive value of MR elastography, an initial clinical application may be to triage patients who are under consideration for biopsy examination to assess possible hepatic fibrosis.
Conflict of interest statement
Conflicts of interests: PJR and RLE and their institution hold patents related to this technology and have a potential financial interest in this research.
Figures





Comment in
-
Stiffness and impedance: the new liver biomarkers.Clin Gastroenterol Hepatol. 2007 Oct;5(10):1144-6. doi: 10.1016/j.cgh.2007.07.024. Clin Gastroenterol Hepatol. 2007. PMID: 17916542 No abstract available.
References
-
- Kim WR, Brown RS, Jr, Terrault NA, El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36:227–242. - PubMed
-
- Shaheen NJ, Hansen RA, Morgan DR, Gangarosa LM, Ringel Y, Thiny MT, Russo MW, et al. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol. 2006;101:2128–2138. - PubMed
-
- Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331–338. - PubMed
-
- Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;2:1048–1058. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

