Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling
- PMID: 17916612
- PMCID: PMC2375462
- DOI: 10.1113/jphysiol.2007.141119
Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling
Abstract
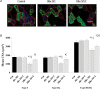
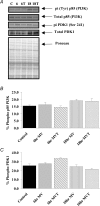
Oxidative stress promotes controlled mechanical ventilation (MV)-induced diaphragmatic atrophy. Nonetheless, the signalling pathways responsible for oxidative stress-induced muscle atrophy remain unknown. We tested the hypothesis that oxidative stress down-regulates insulin-like growth factor-1-phosphotidylinositol 3-kinase-protein kinase B serine threonine kinase (IGF-1-PI3K-Akt) signalling and activates the forkhead box O (FoxO) class of transcription factors in diaphragm fibres during MV-induced diaphragm inactivity. Sprague-Dawley rats were randomly assigned to one of five experimental groups: (1) control (Con), (2) 6 h of MV, (3) 6 h of MV with infusion of the antioxidant Trolox, (4) 18 h of MV, (5) 18 h of MV with Trolox. Following 6 h and 18 h of MV, diaphragmatic Akt activation decreased in parallel with increased nuclear localization and transcriptional activation of FoxO1 and decreased nuclear localization of FoxO3 and FoxO4, culminating in increased expression of the muscle-specific ubiquitin ligases, muscle atrophy factor (MAFbx) and muscle ring finger-1 (MuRF-1). Interestingly, following 18 h of MV, antioxidant administration was associated with attenuation of MV-induced atrophy in type I, type IIa and type IIb/IIx myofibres. Collectively, these data reveal that the antioxidant Trolox attenuates MV-induced diaphragmatic atrophy independent of alterations in Akt regulation of FoxO transcription factors and expression of MAFbx or MuRF-1. Further, these results also indicate that differential regulation of diaphragmatic IGF-1-PI3K-Akt signalling exists during the early and late stages of MV.
Figures




Similar articles
-
Aminophylline Improves Ventilator-Induced Diaphragmatic Dysfunction by Targeting IGF-1-FOXO1-MURF1 Pathway.Discov Med. 2024 Apr;36(183):699-713. doi: 10.24976/Discov.Med.202436183.66. Discov Med. 2024. PMID: 38665019
-
Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness.Crit Care Med. 2011 Jul;39(7):1749-59. doi: 10.1097/CCM.0b013e3182190b62. Crit Care Med. 2011. PMID: 21460706 Free PMC article.
-
A potential role for Akt/FOXO signalling in both protein loss and the impairment of muscle carbohydrate oxidation during sepsis in rodent skeletal muscle.J Physiol. 2008 Nov 15;586(22):5589-600. doi: 10.1113/jphysiol.2008.160150. Epub 2008 Sep 25. J Physiol. 2008. PMID: 18818241 Free PMC article.
-
Signalling pathways that mediate skeletal muscle hypertrophy and atrophy.Nat Cell Biol. 2003 Feb;5(2):87-90. doi: 10.1038/ncb0203-87. Nat Cell Biol. 2003. PMID: 12563267 Review.
-
Signaling pathways perturbing muscle mass.Curr Opin Clin Nutr Metab Care. 2010 May;13(3):225-9. doi: 10.1097/mco.0b013e32833862df. Curr Opin Clin Nutr Metab Care. 2010. PMID: 20397318 Review.
Cited by
-
Levosimendan affects oxidative and inflammatory pathways in the diaphragm of ventilated endotoxemic mice.Crit Care. 2015 Mar 2;19(1):69. doi: 10.1186/s13054-015-0798-8. Crit Care. 2015. PMID: 25888356 Free PMC article.
-
The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals.Aging Cell. 2012 Oct;11(5):801-9. doi: 10.1111/j.1474-9726.2012.00844.x. Epub 2012 Jul 9. Aging Cell. 2012. PMID: 22681576 Free PMC article.
-
Mitochondrial dysfunction induces muscle atrophy during prolonged inactivity: A review of the causes and effects.Arch Biochem Biophys. 2019 Feb 15;662:49-60. doi: 10.1016/j.abb.2018.11.005. Epub 2018 Nov 16. Arch Biochem Biophys. 2019. PMID: 30452895 Free PMC article. Review.
-
Ventilator-induced diaphragm dysfunction in critical illness.Exp Biol Med (Maywood). 2018 Dec;243(17-18):1329-1337. doi: 10.1177/1535370218811950. Epub 2018 Nov 19. Exp Biol Med (Maywood). 2018. PMID: 30453774 Free PMC article. Review.
-
Pressure support ventilation attenuates ventilator-induced protein modifications in the diaphragm.Crit Care. 2008;12(6):191. doi: 10.1186/cc7095. Epub 2008 Nov 7. Crit Care. 2008. PMID: 19040772 Free PMC article.
References
-
- Adams GR, Caiozzo VJ, Baldwin KM. Skeletal muscle unweighting: spaceflight and ground-based models. J Appl Physiol. 2003;95:2185–2201. - PubMed
-
- Betters JL, Criswell DS, Shanely RA, Van Gammeren D, Falk D, Deruisseau KC, Deering M, Yimlamai T, Powers SK. Trolox attenuates mechanical ventilation-induced diaphragmatic dysfunction and proteolysis. Am J Respir Crit Care Med. 2004;170:1179–1184. - PubMed
-
- Bizzozero OA, Reyes S, Ziegler J, Smerjac S. Lipid peroxidation scavengers prevent the carbonylation of cytoskeletal brain proteins induced by glutathione depletion. Neurochem Res. 2007 DOI 10.1007/s11064-007-9377-y in press. - DOI - PubMed
-
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

