Properties of the major classes of mechanoreceptors in the guinea pig bladder
- PMID: 17916614
- PMCID: PMC2375472
- DOI: 10.1113/jphysiol.2007.140244
Properties of the major classes of mechanoreceptors in the guinea pig bladder
Abstract
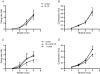
Sensory neurons represent an attractive target for pharmacological treatment of various bladder disorders. However the properties of major classes of mechano-sensory neurons projecting to the bladder have not been systematically established. An in vitro bladder preparation was used to examine the effects of a range of mechanical stimuli (stretch, von Frey hair stroking and focal compression of receptive fields) and chemical stimuli (1 mm alpha,beta-methylene ATP, hypertonic solutions (500 mm NaCl) and 3 microm capsaicin) during electrophysiological recordings from guinea pig bladder afferents. Four functionally distinct populations of bladder sensory neurons were distinguished by these stimuli. The first class, muscle mechanoreceptors, were activated by stretch but not by mucosal stroking with light (0.05-0.1 mN) von Frey hairs or by hypertonic saline, alpha,beta-methylene ATP or capsaicin. Removal of the urothelium did not affect their stretch-induced firing. The second class, muscle-mucosal mechanoreceptors, were activated by both stretch and mucosal stroking with light von Frey hairs or by hypertonic saline and by alpha,beta-methylene ATP, but not by capsaicin. Removal of the urothelium reduced their stretch- and stroking-induced firing. The third class, mucosal high-responding mechanoreceptors, were stretch-insensitive but could be activated by mucosal stroking with light von Frey hairs or by hypertonic saline, alpha,beta-methylene ATP and capsaicin. Stroking-induced firing was significantly reduced by removal of the urothelium. The fourth class, mucosal low-responding mechanoreceptors, were stretch insensitive but could be weakly activated by mucosal stroking with light von Frey hairs but not by hypertonic saline, alpha,beta-methylene ATP or capsaicin. Removal of the urothelium reduced mucosal stroking-induced firing. All four populations of afferents conducted in the C-fibre range and showed class-dependent differences in spike amplitude and duration. At least four functional classes of bladder mechanoreceptors can be readily distinguished by different mechanisms of activation and are likely to transmit different types of information to the central nervous system.
Figures
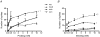








Similar articles
-
Major classes of sensory neurons to the urinary bladder.Auton Neurosci. 2006 Jun 30;126-127:390-7. doi: 10.1016/j.autneu.2006.02.007. Epub 2006 Apr 3. Auton Neurosci. 2006. PMID: 16581309
-
Mechanotransduction and chemosensitivity of two major classes of bladder afferents with endings in the vicinity to the urothelium.J Physiol. 2009 Jul 15;587(Pt 14):3523-38. doi: 10.1113/jphysiol.2009.172577. Epub 2009 May 26. J Physiol. 2009. PMID: 19470774 Free PMC article.
-
Time-of-day dependent changes in guinea pig bladder afferent mechano-sensitivity.Sci Rep. 2021 Sep 29;11(1):19283. doi: 10.1038/s41598-021-98831-x. Sci Rep. 2021. PMID: 34588547 Free PMC article.
-
Mucosal signaling in the bladder.Auton Neurosci. 2016 Oct;200:49-56. doi: 10.1016/j.autneu.2015.08.009. Epub 2015 Sep 24. Auton Neurosci. 2016. PMID: 26422993 Review.
-
The Role of the Mucosa in Normal and Abnormal Bladder Function.Basic Clin Pharmacol Toxicol. 2016 Oct;119 Suppl 3(Suppl 3):57-62. doi: 10.1111/bcpt.12626. Epub 2016 Aug 5. Basic Clin Pharmacol Toxicol. 2016. PMID: 27228303 Free PMC article. Review.
Cited by
-
Activation of MrgprA3 and MrgprC11 on Bladder-Innervating Afferents Induces Peripheral and Central Hypersensitivity to Bladder Distension.J Neurosci. 2021 Apr 28;41(17):3900-3916. doi: 10.1523/JNEUROSCI.0033-21.2021. Epub 2021 Mar 16. J Neurosci. 2021. PMID: 33727332 Free PMC article.
-
Bladder afferent signaling: recent findings.J Urol. 2010 Apr;183(4):1288-95. doi: 10.1016/j.juro.2009.12.060. Epub 2010 Feb 19. J Urol. 2010. PMID: 20171668 Free PMC article. Review.
-
Emerging Families of Ion Channels Involved in Urinary Bladder Nociception.Pharmaceuticals (Basel). 2010 Jul 19;3(7):2248-2267. doi: 10.3390/ph3072248. Pharmaceuticals (Basel). 2010. PMID: 27713353 Free PMC article. Review.
-
[Myofibroblasts and afferent signalling in the urinary bladder. A concept].Urologe A. 2008 Sep;47(9):1085-6, 1088-90. doi: 10.1007/s00120-008-1817-z. Urologe A. 2008. PMID: 18679652 German.
-
Developing a functional urinary bladder: a neuronal context.Front Cell Dev Biol. 2015 Sep 1;3:53. doi: 10.3389/fcell.2015.00053. eCollection 2015. Front Cell Dev Biol. 2015. PMID: 26389118 Free PMC article. Review.
References
-
- Applebaum AE, Vance WH, Coggeshall RE. Segmental localization of sensory cells that innervate the bladder. J Comp Neurol. 1980;192:203–209. - PubMed
-
- Bahns E, Ernsberger U, Jänig W, Nelke A. Functional characteristics of lumbar visceral afferent fibres from the urinary bladder and the urethra in the cat. Pflugers Arch. 1986;407:510–518. - PubMed
-
- Bahns E, Halsband U, Jänig W. Responses of sacral visceral afferents from the lower urinary tract, colon and anus to mechanical stimulation. Pflugers Arch. 1987;410:296–303. - PubMed
-
- Birder LA. More than just a barrier: urothelium as a drug target for urinary bladder pain. Am J Physiol Renal Physiol. 2005;289:F489–F495. - PubMed
-
- Brierley SM, Jones RC, 3rd, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 2004;127:166–178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

