Prostaglandin F2alpha stimulates the expression and secretion of transforming growth factor B1 via induction of the early growth response 1 gene (EGR1) in the bovine corpus luteum
- PMID: 17916653
- PMCID: PMC2234593
- DOI: 10.1210/me.2007-0272
Prostaglandin F2alpha stimulates the expression and secretion of transforming growth factor B1 via induction of the early growth response 1 gene (EGR1) in the bovine corpus luteum
Abstract
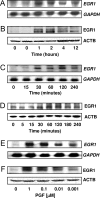
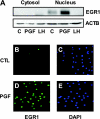
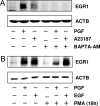
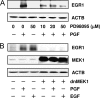
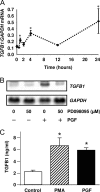
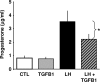
In most mammals, prostaglandin F2alpha (PGF2alpha) is believed to be a trigger that induces the regression of the corpus luteum (CL), whereby progesterone synthesis is inhibited, the luteal structure involutes, and the reproductive cycle resumes. Studies have shown that the early growth response 1 (EGR1) protein can induce the expression of proapoptotic proteins, suggesting that EGR1 may play a role in luteal regression. Our hypothesis is that EGR1 mediates the actions of PGF2alpha by inducing the expression of TGF beta1 (TGFB1), a key tissue remodeling protein. The levels of EGR1 mRNA and protein were up-regulated in the bovine CL during PGF2alpha-induced luteolysis in vivo and in PGF2alpha-treated luteal cells in vitro. Using chemical and genetic approaches, the RAF/MAPK kinase (MEK) 1/ERK pathway was identified as a proximal signaling event required for the induction of EGR1 in PGF2alpha-treated cells. Treatment with PGF2alpha increased the expression of TGFB1 mRNA and protein as well as the binding of EGR1 protein to TGFB1 promoter in bovine luteal cells. The effect of PGF2alpha on TGFB1 expression was mimicked by a protein kinase C (PKC)/RAF/MEK1/ERK activator or adenoviral-mediated expression of EGR1. The stimulatory effect of PGF2alpha on TGFB1 mRNA and TGFB1 protein secretion was inhibited by blockade of MEK1/ERK signaling and by adenoviral-mediated expression of NAB2, an EGR1 binding protein that inhibits EGR1 transcriptional activity. Treatment of luteal cells with TGFB1 reduced progesterone secretion, implicating TGFB1 in luteal regression. These studies demonstrate that PGF2alpha stimulates the expression of EGR1 and TGFB1 in the CL. We suggest that EGR1 plays a role in the expression of genes whose cognate proteins coordinate luteal regression.
Figures
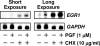



References
-
- Davis JS, Rueda BR 2002 The corpus luteum: an ovarian structure with maternal instincts and suicidal tendencies. Front Biosci 7:d1949–d1978 - PubMed
-
- Stocco C, Telleria C, Gibori G 2007 The molecular control of corpus luteum formation, function, and regression. Endocr Rev 28:117–149 - PubMed
-
- Sakamoto K, Ezashi T, Miwa K, Okuda-Ashitaka E, Houtani T, Sugimoto T, Ito S, Hayaishi O 1994 Molecular cloning and expression of a cDNA of the bovine prostaglandin F2 α receptor. J Biol Chem 269:3881–3886 - PubMed
-
- Davis JS 1987 Stimulation of intracellular free Ca2+ by luteinizing hormone in isolated bovine luteal cells. Adv Exp Med Biol 219:671–675 - PubMed
-
- Chen DB, Westfall SD, Fong HW, Roberson MS, Davis JS 1998 Prostaglandin F2α stimulates the Raf/MEK1/mitogen-activated protein kinase signaling cascade in bovine luteal cells. Endocrinology 139:3876–3885 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

