Fibroblast growth factor receptor-1 signaling in pancreatic islet beta-cells is modulated by the extracellular matrix
- PMID: 17916654
- PMCID: PMC2194636
- DOI: 10.1210/me.2007-0241
Fibroblast growth factor receptor-1 signaling in pancreatic islet beta-cells is modulated by the extracellular matrix
Abstract
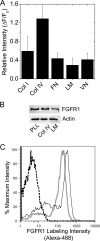
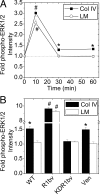
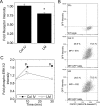
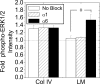
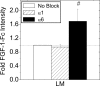
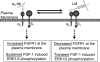
Maintenance of pancreatic beta-cell mass depends on extracellular stimuli that promote survival and proliferation. In the islet, these stimuli come from the beta-cell microenvironment and include extracellular matrix deposited by associated vascular endothelial cells. Fibroblast growth factor receptor-1 (FGFR1) has recently been implicated as a signaling pathway that is important for normal beta-cell function. We would like to understand how extracellular matrix and FGFR1 signaling interact to promote beta-cell survival and proliferation. To examine beta-cell-specific receptor responses, we created lentiviral vectors with rat insulin promoter-driven expression of Venus fluorescent protein-tagged full-length (R1betav) and kinase-deficient (KDR1betav) FGFR1. Significant FGF-1-dependent activation of ERK1/2 was observed in betaTC3 cells, dispersed beta-cells, and beta-cells in intact islets. This response was enhanced by R1betav expression and reduced by KDR1betav expression. Plating-dispersed beta-cells on collagen type IV resulted in enhanced expression of endogenous FGFR1 that was associated with sustained activation of ERK1/2. Conversely, plating cells on laminin reduced expression of FGFR1, and this reduction was associated with transient activation of ERK1/2. Addition of neutralizing antibodies to inhibit beta-cell attachment to laminin via alpha(6)-integrin increased high-affinity FGF-1-binding at the plasma membrane and resulted in sustained ERK1/2 activity similar to cells plated on collagen type IV. These data show that the FGF-stimulated beta-cell response is negatively affected by alpha(6)-integrin binding to laminin and suggest regulation associated with vascular endothelial cell remodeling.
Figures
References
-
- Gospodarowicz D, Cheng J 1986 Heparin protects basic and acidic FGF from inactivation. J Cell Physiol 128:475–484 - PubMed
-
- Johnson DE, Williams LT 1993 Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res 60:1–41 - PubMed
-
- Cool SM, Sayer RE, van Heumen WR, Pickles JO, Nurcombe V 2002 Temporal and spatial expression of fibroblast growth factor receptor 4 isoforms in murine tissues. Histochem J 34:291–297 - PubMed
-
- Arany E, Hill DJ 2000 Ontogeny of fibroblast growth factors in the early development of the rat endocrine pancreas. Pediatr Res 48:389–403 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK58404/DK/NIDDK NIH HHS/United States
- K01 DK068206/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- P30 HD015052/HD/NICHD NIH HHS/United States
- EY08126/EY/NEI NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- R01 DK053434/DK/NIDDK NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- HD15052/HD/NICHD NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- P60 DK020593/DK/NIDDK NIH HHS/United States
- DK68206/DK/NIDDK NIH HHS/United States
- DK59637/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- DK53434/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

