Defective protein folding and intracellular retention of thyroglobulin-R19K mutant as a cause of human congenital goiter
- PMID: 17916655
- PMCID: PMC2725791
- DOI: 10.1210/me.2007-0183
Defective protein folding and intracellular retention of thyroglobulin-R19K mutant as a cause of human congenital goiter
Abstract
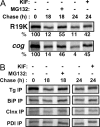
It has been suggested that a thyroglobulin (Tg)-R19K missense mutation may be a newly identified cause of human congenital goiter, which is surprising for this seemingly conservative substitution. Here, we have examined the intracellular fate of recombinant mutant Tg expressed in COS-7 cells. Incorporation of the R19K mutation largely blocked Tg secretion, and this mutant was approximately 90% degraded intracellularly over a 24-h period after synthesis. Before its degradation, the Tg-R19K mutant exhibited abnormally increased association with molecular chaperones BiP, calnexin, and protein disulfide isomerase, and was unable to undergo anterograde advance from the endoplasmic reticulum (ER) through the Golgi complex. Inhibitors of proteasomal proteolysis and ER mannosidase-I both prevented ER-associated degradation of the Tg-R19K mutant and increased its association with ER molecular chaperones. ER quality control around Tg residue 19 is not dependent upon charge but upon side-chain packing, because Tg-R19Q was efficiently secreted. Whereas a Tg mutant truncated after residue 174 folds sufficiently well to escape ER quality control, introduction of the R19K point mutation blocked its secretion. The data indicate that the R19K mutation induces local misfolding in the amino-terminal domain of Tg that has global effects on Tg transport and thyroid hormonogenesis.
Figures




Similar articles
-
Enhanced binding to the molecular chaperone BiP slows thyroglobulin export from the endoplasmic reticulum.Mol Endocrinol. 1998 Mar;12(3):458-67. doi: 10.1210/mend.12.3.0069. Mol Endocrinol. 1998. PMID: 9514162
-
Unfolded protein response is involved in the pathology of human congenital hypothyroid goiter and rat non-goitrous congenital hypothyroidism.J Mol Endocrinol. 2004 Jun;32(3):903-20. doi: 10.1677/jme.0.0320903. J Mol Endocrinol. 2004. PMID: 15171721
-
Mixed-disulfide folding intermediates between thyroglobulin and endoplasmic reticulum resident oxidoreductases ERp57 and protein disulfide isomerase.Mol Cell Biol. 2005 Nov;25(22):9793-805. doi: 10.1128/MCB.25.22.9793-9805.2005. Mol Cell Biol. 2005. PMID: 16260597 Free PMC article.
-
Thyroglobulin From Molecular and Cellular Biology to Clinical Endocrinology.Endocr Rev. 2016 Feb;37(1):2-36. doi: 10.1210/er.2015-1090. Epub 2015 Nov 23. Endocr Rev. 2016. PMID: 26595189 Free PMC article. Review.
-
Protein folding and quality control in the endoplasmic reticulum.Curr Opin Cell Biol. 2004 Aug;16(4):343-9. doi: 10.1016/j.ceb.2004.06.012. Curr Opin Cell Biol. 2004. PMID: 15261665 Review.
Cited by
-
Proteostasis strategies for restoring alpha1-antitrypsin deficiency.Proc Am Thorac Soc. 2010 Nov;7(6):415-22. doi: 10.1513/pats.201001-016AW. Proc Am Thorac Soc. 2010. PMID: 21030523 Free PMC article. Review.
-
Functions of the Thyroid-Stimulating Hormone on Key Developmental Features Revealed in a Series of Zebrafish Dyshormonogenesis Models.Cells. 2021 Aug 4;10(8):1984. doi: 10.3390/cells10081984. Cells. 2021. PMID: 34440752 Free PMC article.
-
GRP94: An HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum.Biochim Biophys Acta. 2012 Mar;1823(3):774-87. doi: 10.1016/j.bbamcr.2011.10.013. Epub 2011 Nov 3. Biochim Biophys Acta. 2012. PMID: 22079671 Free PMC article. Review.
-
Maturation of thyroglobulin protein region I.J Biol Chem. 2011 Sep 23;286(38):33045-52. doi: 10.1074/jbc.M111.281337. Epub 2011 Aug 4. J Biol Chem. 2011. PMID: 21816825 Free PMC article.
-
Loss-of-function PCSK9 mutants evade the unfolded protein response sensor GRP78 and fail to induce endoplasmic reticulum stress when retained.J Biol Chem. 2018 May 11;293(19):7329-7343. doi: 10.1074/jbc.RA117.001049. Epub 2018 Mar 28. J Biol Chem. 2018. PMID: 29593095 Free PMC article.
References
-
- Di Jeso B, Arvan P 2004 Thyroglobulin structure, function, and biosynthesis. Chap 5. In: Braverman LE, Utiger R, eds. The thyroid. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 77–95
-
- Dunn JT, Dunn AD 1999 The importance of thyroglobulin structure for thyroid hormone biosynthesis. Biochimie 81:505–509 - PubMed
-
- Arvan P, Kim PS, Kuliawat R, Prabakaran D, Muresan Z, Yoo SE, Hossain SA 1997 Intracellular protein transport to the thyrocyte plasma membrane: potential implications for thyroid physiology. Thyroid 7:89–105 - PubMed
-
- Kim PS, Arvan P 1993 Hormonal regulation of thyroglobulin export from the endoplasmic reticulum of cultured thyrocytes. J Biol Chem 268:4873–4879 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

