Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses
- PMID: 17916800
- PMCID: PMC2757778
- DOI: 10.1634/stemcells.2007-0563
Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses
Abstract
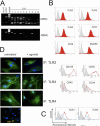
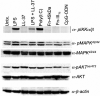
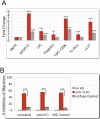
Adult human bone marrow-derived mesenchymal stem cells (hMSCs) are under study as therapeutic delivery agents that assist in the repair of damaged tissues. To achieve the desired clinical outcomes for this strategy requires a better understanding of the mechanisms that drive the recruitment, migration, and engraftment of hMSCs to the targeted tissues. It is known that hMSCs are recruited to sites of stress or inflammation to fulfill their repair function. It is recognized that toll-like receptors (TLRs) mediate stress responses of other bone marrow-derived cells. This study explored the role of TLRs in mediating stress responses of hMSCs. Accordingly, the presence of TLRs in hMSCs was initially established by reverse transcription-polymerase chain reaction assays. Flow cytometry and fluorescence immunocytochemical analyses confirmed these findings. The stimulation of hMSCs with TLR agonists led to the activation of downstream signaling pathways, including nuclear factor kappaB, AKT, and MAPK. Consequently, activation of these pathways triggered the induction and secretion of cytokines, chemokines, and related TLR gene products as established from cDNA array, immunoassay, and cytokine antibody array analyses. Interestingly, the unique patterns of affected genes, cytokines, and chemokines measured identify these receptors as critical players in the clinically established immunomodulation observed for hMSCs. Lastly, hMSC migration was promoted by TLR ligand exposure as demonstrated by transwell migration assays. Conversely, disruption of TLRs by neutralizing TLR antibodies compromised hMSC migration. This study defines a novel TLR-driven stress and immune modulating response for hMSCs that is critical to consider in the design of stem cell-based therapies.
Figures

References
-
- West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu Rev Cell Dev Biol. 2006;22:409–437. - PubMed
-
- Sabroe I, Read RC, Whyte MK, Dockrell DH, Vogel SN, Dower SK. Toll-like receptors in health and disease: complex questions remain. J Immunol. 2003 Aug 15;171(4):1630–1635. - PubMed
-
- Miggin SM, O'Neill LA. New insights into the regulation of TLR signaling. J Leukoc Biol. 2006 Aug;80(2):220–226. - PubMed
-
- Anders HJ, Banas B, Schlondorff D. Signaling danger: toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol. 2004 Apr;15(4):854–867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

