Acquired immunologic tolerance: with particular reference to transplantation
- PMID: 17917005
- PMCID: PMC2800371
- DOI: 10.1007/s12026-007-0001-7
Acquired immunologic tolerance: with particular reference to transplantation
Abstract
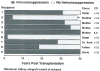
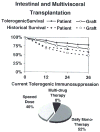
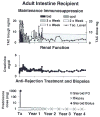
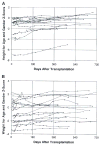
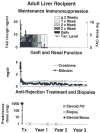
The first unequivocally successful bone marrow cell transplantation in humans was recorded in 1968 by the University of Minnesota team of Robert A. Good (Gatti et al. Lancet 2: 1366-1369, 1968). This achievement was a direct extension of mouse models of acquired immunologic tolerance that were established 15 years earlier. In contrast, organ (i.e. kidney) transplantation was accomplished precociously in humans (in 1959) before demonstrating its feasibility in any experimental model and in the absence of a defensible immunologic rationale. Due to the striking differences between the outcomes with the two kinds of procedure, the mechanisms of organ engraftment were long thought to differ from the leukocyte chimerism-associated ones of bone marrow transplantation. This and other concepts of alloengraftment and acquired tolerance have changed over time. Current concepts and their clinical implications can be understood and discussed best from the perspective provided by the life and times of Bob Good.
Figures











Similar articles
-
Transplantation tolerance from a historical perspective.Nat Rev Immunol. 2001 Dec;1(3):233-9. doi: 10.1038/35105088. Nat Rev Immunol. 2001. PMID: 11905833 Free PMC article. Review.
-
Transient mixed chimerism for allograft tolerance.Chimerism. 2015 Apr 3;6(1-2):21-6. doi: 10.1080/19381956.2015.1111975. Epub 2015 Oct 30. Chimerism. 2015. PMID: 26517761 Free PMC article. Review.
-
The birth of clinical organ transplantation.J Am Coll Surg. 2001 Apr;192(4):431-46. doi: 10.1016/s1072-7515(01)00804-3. J Am Coll Surg. 2001. PMID: 11294399 Free PMC article. No abstract available.
-
Path to clinical transplantation tolerance and prevention of graft-versus-host disease.Immunol Res. 2014 May;58(2-3):240-8. doi: 10.1007/s12026-014-8502-7. Immunol Res. 2014. PMID: 24671802 Free PMC article. Review.
-
[Transplantation surgery and immunology: from origins to new paradigms of immune tolerance].Bull Mem Acad R Med Belg. 2002;157(10-12):439-44; discussion 444-7. Bull Mem Acad R Med Belg. 2002. PMID: 12854184 French.
Cited by
-
Immunosuppressive therapy and tolerance of organ allografts.N Engl J Med. 2008 Jan 24;358(4):407-11. doi: 10.1056/NEJMe0707578. N Engl J Med. 2008. PMID: 18216363 Free PMC article. No abstract available.
-
Complications of right lobe living donor liver transplantation.J Hepatol. 2009 Oct;51(4):715-24. doi: 10.1016/j.jhep.2009.04.023. Epub 2009 May 27. J Hepatol. 2009. PMID: 19576652 Free PMC article.
-
Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression.Ann Surg. 2013 Feb;257(2):345-51. doi: 10.1097/SLA.0b013e31826d90bb. Ann Surg. 2013. PMID: 23001085 Free PMC article.
-
Donor-derived peripheral mononuclear cell DNA is associated with stable kidney allograft function: a randomized controlled trial.Chimerism. 2011 Oct-Dec;2(4):102-10. doi: 10.4161/chim.2.4.19095. Chimerism. 2011. PMID: 22509426 Free PMC article. Clinical Trial.
-
Anthony Cerami Award in Translational Medicine: A Journey in Science: The Birth of Organ Transplantation with Particular Reference to Alloengraftment Mechanisms.Mol Med. 2015 Jun 25;21(1):227-32. doi: 10.2119/molmed.2014.00254. Mol Med. 2015. PMID: 26197024 Free PMC article. No abstract available.
References
-
- Billingham RE, Brent L, Medawar PB. “Actively acquired tolerance” of foreign cells. Nature. 1953;172:603–6. - PubMed
-
- Billingham R, Brent L, Medawar P. Quantitative studies on tissue transplantation immunity. III. Actively acquired tolerance. Philos Trans R Soc Lond (Biol) 1956;239:357–412.
-
- Main JM, Prehn RT. Successful skin homografts after the administration of high dosage X radiation and homologous bone marrow. J Natl Cancer Inst. 1955;15:1023–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

