Heat shock proteins and amateur chaperones in amyloid-Beta accumulation and clearance in Alzheimer's disease
- PMID: 17917109
- PMCID: PMC2039847
- DOI: 10.1007/s12035-007-0029-7
Heat shock proteins and amateur chaperones in amyloid-Beta accumulation and clearance in Alzheimer's disease
Abstract
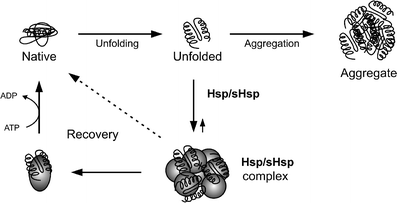
The pathologic lesions of Alzheimer's disease (AD) are characterized by accumulation of protein aggregates consisting of intracellular or extracellular misfolded proteins. The amyloid-beta (Abeta) protein accumulates extracellularly in senile plaques and cerebral amyloid angiopathy, whereas the hyperphosphorylated tau protein accumulates intracellularly as neurofibrillary tangles. "Professional chaperones", such as the heat shock protein family, have a function in the prevention of protein misfolding and subsequent aggregation. "Amateur" chaperones, such as apolipoproteins and heparan sulfate proteoglycans, bind amyloidogenic proteins and may affect their aggregation process. Professional and amateur chaperones not only colocalize with the pathological lesions of AD, but may also be involved in conformational changes of Abeta, and in the clearance of Abeta from the brain via phagocytosis or active transport across the blood-brain barrier. Thus, both professional and amateur chaperones may be involved in the aggregation, accumulation, persistence, and clearance of Abeta and tau and in other Abeta-associated reactions such as inflammation associated with AD lesions, and may, therefore, serve as potential targets for therapeutic intervention.
Figures
Similar articles
-
Do amyloid β-associated factors co-deposit with Aβ in mouse models for Alzheimer's disease?J Alzheimers Dis. 2010;22(2):345-55. doi: 10.3233/JAD-2010-100711. J Alzheimers Dis. 2010. PMID: 20847441 Review.
-
Small heat shock protein HspB8: its distribution in Alzheimer's disease brains and its inhibition of amyloid-beta protein aggregation and cerebrovascular amyloid-beta toxicity.Acta Neuropathol. 2006 Feb;111(2):139-49. doi: 10.1007/s00401-005-0030-z. Epub 2006 Feb 17. Acta Neuropathol. 2006. PMID: 16485107
-
Amyloid-β oligomers are sequestered by both intracellular and extracellular chaperones.Biochemistry. 2012 Nov 20;51(46):9270-6. doi: 10.1021/bi301277k. Epub 2012 Nov 8. Biochemistry. 2012. PMID: 23106396 Free PMC article.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Collagen XVIII: a novel heparan sulfate proteoglycan associated with vascular amyloid depositions and senile plaques in Alzheimer's disease brains.Brain Pathol. 2002 Oct;12(4):456-62. doi: 10.1111/j.1750-3639.2002.tb00462.x. Brain Pathol. 2002. PMID: 12408231 Free PMC article.
Cited by
-
Vitamin D-binding protein interacts with Aβ and suppresses Aβ-mediated pathology.Cell Death Differ. 2013 Apr;20(4):630-8. doi: 10.1038/cdd.2012.161. Epub 2012 Dec 21. Cell Death Differ. 2013. PMID: 23257976 Free PMC article.
-
Cell-derived soluble oligomers of human amyloid-beta peptides disturb cellular homeostasis and induce apoptosis in primary hippocampal neurons.J Neural Transm (Vienna). 2009 Dec;116(12):1561-9. doi: 10.1007/s00702-009-0311-0. Epub 2009 Oct 7. J Neural Transm (Vienna). 2009. PMID: 19809865
-
The amyloid interactome: Exploring protein aggregation.PLoS One. 2017 Mar 1;12(3):e0173163. doi: 10.1371/journal.pone.0173163. eCollection 2017. PLoS One. 2017. PMID: 28249044 Free PMC article.
-
Potential synergy between tau aggregation inhibitors and tau chaperone modulators.Alzheimers Res Ther. 2013 Sep 16;5(5):41. doi: 10.1186/alzrt207. eCollection 2013. Alzheimers Res Ther. 2013. PMID: 24041111 Free PMC article. Review.
-
Immunotherapeutic approaches for Alzheimer's disease in transgenic mouse models.Brain Struct Funct. 2010 Mar;214(2-3):201-18. doi: 10.1007/s00429-009-0236-2. Epub 2009 Dec 10. Brain Struct Funct. 2010. PMID: 20012091 Free PMC article. Review.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '1673054', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/1673054/'}]}
- Selkoe DJ (1991) The molecular pathology of Alzheimer’s disease. Neuron 6:487–498 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '6375662', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/6375662/'}]}
- Glenner GG, Wong CW (1984) Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120:885–890 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '1899488', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/1899488/'}]}
- Lee VM, Balin BJ, Otvos L, Jr, Trojanowski JQ (1991) A68: a major subunit of paired helical filaments and derivatized forms of normal tau. Science 251:675–678 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '15140180', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15140180/'}]}
- Zlokovic BV (2004) Clearing amyloid through the blood-brain barrier. J Neurochem 89:807–811 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '12706236', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12706236/'}]}
- Nagele RG, D’Andrea MR, Lee H, Venkataraman V, Wang HY (2003) Astrocytes accumulate A beta 42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Res 971:197–209 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

