Inhibitors of the interaction of a thyroid hormone receptor and coactivators: preliminary structure-activity relationships
- PMID: 17918822
- PMCID: PMC2536611
- DOI: 10.1021/jm070556y
Inhibitors of the interaction of a thyroid hormone receptor and coactivators: preliminary structure-activity relationships
Abstract
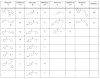
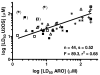
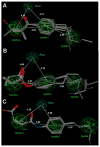
The modulation of gene regulation by blocking the interaction between the thyroid receptor (TR) and obligate coregulators has been reported recently with the discovery of the lead compound 3-(dimethylamino)-1-(4-hexylphenyl)propan-1-one). Herein we report studies aimed at optimization of this initial hit to determine the basic parameters of the structure-activity relationships and clarify the mechanism of action. These studies provided new insights, showing that activity and TRbeta isoform selectivity is highly correlated with the structural composition of these covalent inhibitors.
Figures



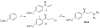

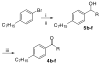

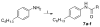


References
-
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81(3):1097–1142. - PubMed
-
- Malm J. Thyroid hormone ligands and metabolic diseases. Curr Pharm Des. 2004;10(28):3525–3532. - PubMed
-
- Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81(3):1269–1304. - PubMed
-
- Harvey CB, Williams GR. Mechanism of thyroid hormone action. Thyroid. 2002;12(6):441–446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

