Use of docking peptides to design modular substrates with high efficiency for mitogen-activated protein kinase extracellular signal-regulated kinase
- PMID: 17918909
- PMCID: PMC2597387
- DOI: 10.1021/cb700158q
Use of docking peptides to design modular substrates with high efficiency for mitogen-activated protein kinase extracellular signal-regulated kinase
Abstract
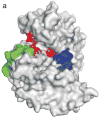
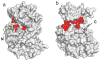
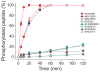
The mitogen-activated protein kinase extracellular regulated kinase (ERK) plays a key role in the regulation of cellular proliferation. Mutations in the ERK cascade occur in 30% of malignant tumors. Thus understanding how the kinase identifies its cognate substrates as well as monitoring the activity of ERK is central to cancer research and therapeutic development. ERK binds to its protein targets, both downstream substrates and upstream activators, via a binding site distinct from the catalytic site of ERK. The substrate sequences that bind, or dock, to these sites on ERK influence the efficiency of phosphorylation. For this reason, simple peptide substrates containing only phosphorylation sequences typically possess low efficiencies for ERK. Appending short docking peptides derived from full-length protein substrates and activators of ERK to a phosphorylation sequence increased the affinity of ERK for the phosphorylation sequence by as much as 200-fold while only slightly diminishing the maximal velocity of the reaction. The efficiency of the phosphorylation reaction was increased by up to 150-fold, while the specificity of the substrate for ERK was preserved. Simple modular peptide substrates, which can be easily tailored to possess high phosphorylation efficiencies, will enhance our understanding of the regulation of ERK and provide a tool for the development of new kinase assays.
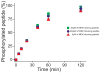
Figures

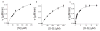
References
-
- Cobb MH. MAP kinase pathways. Progress in Biophysics and Molecular Biology. 1999;71:479–500. - PubMed
-
- Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. - PubMed
-
- Bradham C, McClay DR. p38 MAPK in development and cancer. Cell Cycle. 2006;5:8. - PubMed
-
- Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochimica et Biophysica Acta (BBA) - Proteins & Proteomics. 2005;1754:253–262. - PubMed
-
- Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: from junk to gold? Nature Reviews Drug Discovery. 2003;2:554–565. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

