Computational studies of the farnesyltransferase ternary complex part II: the conformational activation of farnesyldiphosphate
- PMID: 17918965
- PMCID: PMC2570014
- DOI: 10.1021/bi701324t
Computational studies of the farnesyltransferase ternary complex part II: the conformational activation of farnesyldiphosphate
Abstract
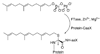
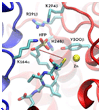
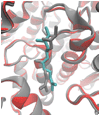
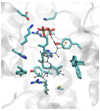
Studies aimed at elucidating the reaction mechanism of farnesyltransferase (FTase), which catalyzes the prenylation of many cellular signaling proteins including Ras, has been an active area of research. Much is known regarding substrate binding and the impact of various catalytic site residues on catalysis. However, the molecular level details regarding the conformational rearrangement of farnesyldiphosphate (FPP), which has been proposed via structural analysis and mutagenesis studies to occur prior to the chemical step, is still poorly understood. Following on our previous computational characterization of the resting state of the FTase ternary complex, the thermodynamics of the conformational rearrangement step in the absence of magnesium was investigated for the wild type FTase and the Y300Fbeta mutant complexed with the peptide CVIM. In addition, we also explored the target dependence of the conformational activation step by perturbing isoleucine into a leucine (CVLM). The calculated free energy profiles of the proposed conformational transition confirm the presence of a stable intermediate state, which was identified only when the diphosphate is monoprotonated (FPP2-). The farnesyl group in the computed intermediate state assumes a conformation similar to that of the product complex, particularly for the first two isoprene units. We found that Y300beta can readily form hydrogen bonds with either of the phosphates of FPP. Removing the hydroxyl group on Y300beta does not significantly alter the thermodynamics of the conformational transition, but shifts the location of the intermediate farther away from the nucleophile by 0.5 A, which suggests that Y300beta facilitate the reaction by stabilizing the chemical step. Our results also showed an increased transition barrier height for CVLM (1.5 kcal/mol higher than that of CVIM). Although qualitatively consistent with the findings from the recent kinetic isotope experiments by Fierke and co-workers, the magnitude is not large enough to affect the rate-limiting step.
Figures

Similar articles
-
Finding a needle in the haystack: computational modeling of Mg2+ binding in the active site of protein farnesyltransferase.Biochemistry. 2010 Nov 9;49(44):9658-66. doi: 10.1021/bi1008358. Biochemistry. 2010. PMID: 20923173 Free PMC article.
-
Kinetic studies of protein farnesyltransferase mutants establish active substrate conformation.Biochemistry. 2003 Aug 19;42(32):9741-8. doi: 10.1021/bi0346852. Biochemistry. 2003. PMID: 12911316
-
Computational studies of the farnesyltransferase ternary complex part I: substrate binding.Biochemistry. 2005 Dec 20;44(50):16513-23. doi: 10.1021/bi051020m. Biochemistry. 2005. PMID: 16342942 Free PMC article.
-
Photoactive analogs of farnesyl diphosphate and related isoprenoids: design and applications in studies of medicinally important isoprenoid-utilizing enzymes.Curr Med Chem. 2013;20(12):1585-94. doi: 10.2174/0929867311320120008. Curr Med Chem. 2013. PMID: 23409719 Review.
-
Protein farnesyltransferase inhibitors.Curr Med Chem. 2002 May;9(10):1003-32. doi: 10.2174/0929867024606687. Curr Med Chem. 2002. PMID: 12733981 Review.
Cited by
-
Molecular dynamics analysis of a series of 22 potential farnesyltransferase substrates containing a CaaX-motif.J Mol Model. 2013 Feb;19(2):673-88. doi: 10.1007/s00894-012-1590-1. Epub 2012 Sep 26. J Mol Model. 2013. PMID: 23011608
-
Insights into the mechanistic dichotomy of the protein farnesyltransferase peptide substrates CVIM and CVLS.J Am Chem Soc. 2012 Jan 18;134(2):820-3. doi: 10.1021/ja209650h. Epub 2012 Jan 6. J Am Chem Soc. 2012. PMID: 22206225 Free PMC article.
-
Catalytic mechanism of aromatic prenylation by NphB.Biochemistry. 2012 Mar 27;51(12):2606-18. doi: 10.1021/bi201800m. Epub 2012 Mar 12. Biochemistry. 2012. PMID: 22385275 Free PMC article.
-
Protein farnesyltransferase-catalyzed isoprenoid transfer to peptide depends on lipid size and shape, not hydrophobicity.Chembiochem. 2008 Nov 24;9(17):2872-82. doi: 10.1002/cbic.200800248. Chembiochem. 2008. PMID: 18985644 Free PMC article.
-
Simultaneous Site-Specific Dual Protein Labeling Using Protein Prenyltransferases.Bioconjug Chem. 2015 Dec 16;26(12):2542-53. doi: 10.1021/acs.bioconjchem.5b00553. Epub 2015 Dec 4. Bioconjug Chem. 2015. PMID: 26561785 Free PMC article.
References
-
- Adjei AA. Blocking oncogenic Ras signaling for cancer therapy. J. Natl. Cancer I. 2001;93:1062–1074. - PubMed
-
- Resh M. Regulation of cellular signaling by fatty acid acylation and prenylation of signal transduction proteins. Cell. Signaling. 1996;8:403–412. - PubMed
-
- Roskoski R. Protein prenylation: a pivotal posttranslational process. Biochem. Bioph. Res. Co. 2003;303:1–7. - PubMed
-
- Seabra M. Membrane association and targeting of prenylated Ras-like GTPases. Cell. Signaling. 1998;10:167–172. - PubMed
-
- Sinensky M. Recent advances in the study of prenylated proteins. Bba-Mol. Cell. Biol. L. 2000;1484:93–106. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

