TCF4 deficiency expands ventral diencephalon signaling and increases induction of pituitary progenitors
- PMID: 17919533
- PMCID: PMC2693283
- DOI: 10.1016/j.ydbio.2007.08.046
TCF4 deficiency expands ventral diencephalon signaling and increases induction of pituitary progenitors
Abstract
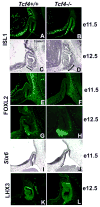
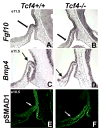
The anterior and intermediate lobes of the pituitary gland are formed from Rathke's pouch. FGF, BMP and WNT signals emanating from the ventral diencephalon influence pouch growth and development. In order to examine the role of canonical WNT signaling during pituitary development we examined the pituitary expression of the TCF/LEF family of transcription factors, which mediate WNT signaling through the binding of beta-catenin. We report here the expression of several members of this family during pituitary development and the functional role of one member, TCF4 (TCF7L2), in the induction of the pituitary primordium. TCF4 is expressed in the ventral diencephalon early in pituitary development, rostral to a domain of BMP and FGF expression. Tcf4 deficient mice express Fgf10 and Bmp4; however, the Bmp and Fgf expression domains are expanded rostrally. As a result, additional pituitary progenitor cells are recruited into Rathke's pouch in Tcf4 mutants. Mutants also exhibit an expansion of the Six6 expression domain within Rathke's pouch, which may increase the number of proliferating pouch cells, resulting in a greatly enlarged anterior pituitary gland. This suggests that TCF4 negatively regulates pituitary growth through two mechanisms. The first mechanism is to restrict the domains of BMP and FGF signaling in the ventral diencephalon, and the second mechanism is the restriction of Six6 within Rathke's pouch. Thus, TCF4 is necessary both intrinsically and extrinsically to Rathke's pouch to ensure the proper growth of the pituitary gland.
Figures






References
-
- Basch M, Bronner-Fraser M. Neural crest inducing signals. Adv Exp Med Biol. 2006;589:34–31. - PubMed
-
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–78. - PubMed
-
- Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development. 1996;122:1693–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

