Measurements of single DNA molecule packaging dynamics in bacteriophage lambda reveal high forces, high motor processivity, and capsid transformations
- PMID: 17919653
- PMCID: PMC3311920
- DOI: 10.1016/j.jmb.2007.09.011
Measurements of single DNA molecule packaging dynamics in bacteriophage lambda reveal high forces, high motor processivity, and capsid transformations
Abstract
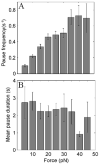
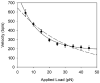
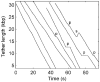
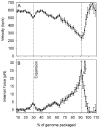
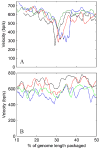
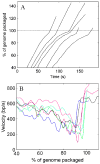
Molecular motors drive genome packaging into preformed procapsids in many double-stranded (ds)DNA viruses. Here, we present optical tweezers measurements of single DNA molecule packaging in bacteriophage lambda. DNA-gpA-gpNu1 complexes were assembled with recombinant gpA and gpNu1 proteins and tethered to microspheres, and procapsids were attached to separate microspheres. DNA binding and initiation of packaging were observed within a few seconds of bringing these microspheres into proximity in the presence of ATP. The motor was observed to generate greater than 50 picoNewtons (pN) of force, in the same range as observed with bacteriophage phi29, suggesting that high force generation is a common property of viral packaging motors. However, at low capsid filling the packaging rate averaged approximately 600 bp/s, which is 3.5-fold higher than phi29, and the motor processivity was also threefold higher, with less than one slip per genome length translocated. The packaging rate slowed significantly with increasing capsid filling, indicating a buildup of internal force reaching 14 pN at 86% packaging, in good agreement with the force driving DNA ejection measured in osmotic pressure experiments and calculated theoretically. Taken together, these experiments show that the internal force that builds during packaging is largely available to drive subsequent DNA ejection. In addition, we observed an 80 bp/s dip in the average packaging rate at 30% packaging, suggesting that procapsid expansion occurs at this point following the buildup of an average of 4 pN of internal force. In experiments with a DNA construct longer than the wild-type genome, a sudden acceleration in packaging rate was observed above 90% packaging, and much greater than 100% of the genome length was translocated, suggesting that internal force can rupture the immature procapsid, which lacks an accessory protein (gpD).
Figures
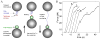
Similar articles
-
Packaging of a unit-length viral genome: the role of nucleotides and the gpD decoration protein in stable nucleocapsid assembly in bacteriophage lambda.J Mol Biol. 2008 Nov 28;383(5):1037-48. doi: 10.1016/j.jmb.2008.08.063. Epub 2008 Sep 3. J Mol Biol. 2008. PMID: 18801370
-
Single-Molecule Measurements of Motor-Driven Viral DNA Packaging in Bacteriophages Phi29, Lambda, and T4 with Optical Tweezers.Methods Mol Biol. 2018;1805:393-422. doi: 10.1007/978-1-4939-8556-2_20. Methods Mol Biol. 2018. PMID: 29971729
-
Methods for Studying Motor-Driven Viral DNA Packaging in Bacteriophages phi29, Lambda, and T4 via Single DNA Molecule Manipulation and Rapid Solution Exchange.Methods Mol Biol. 2025;2881:293-327. doi: 10.1007/978-1-0716-4280-1_15. Methods Mol Biol. 2025. PMID: 39704950
-
Viral genome packaging machines: Structure and enzymology.Enzymes. 2021;50:369-413. doi: 10.1016/bs.enz.2021.09.006. Epub 2021 Nov 10. Enzymes. 2021. PMID: 34861943 Review.
-
The DNA-packaging nanomotor of tailed bacteriophages.Nat Rev Microbiol. 2011 Aug 12;9(9):647-57. doi: 10.1038/nrmicro2632. Nat Rev Microbiol. 2011. PMID: 21836625 Review.
Cited by
-
Dualities in the analysis of phage DNA packaging motors.Bacteriophage. 2012 Oct 1;2(4):239-255. doi: 10.4161/bact.23829. Bacteriophage. 2012. PMID: 23532204 Free PMC article.
-
Keeping It Together: Structures, Functions, and Applications of Viral Decoration Proteins.Viruses. 2020 Oct 14;12(10):1163. doi: 10.3390/v12101163. Viruses. 2020. PMID: 33066635 Free PMC article. Review.
-
A hypothesis for bacteriophage DNA packaging motors.Viruses. 2010 Sep;2(9):1821-1843. doi: 10.3390/v2091821. Epub 2010 Aug 26. Viruses. 2010. PMID: 21994710 Free PMC article.
-
Portal control of viral prohead expansion and DNA packaging.Virology. 2009 Aug 15;391(1):44-50. doi: 10.1016/j.virol.2009.05.029. Epub 2009 Jun 21. Virology. 2009. PMID: 19541336 Free PMC article.
-
Genome packaging in viruses.Curr Opin Struct Biol. 2010 Feb;20(1):114-20. doi: 10.1016/j.sbi.2009.12.006. Epub 2010 Jan 8. Curr Opin Struct Biol. 2010. PMID: 20060706 Free PMC article. Review.
References
-
- Hendrix RW, Roberts JW, Stahl FW, Weisberg RA. Lambda II. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y: 1983.
-
- Murialdo H. Bacteriophage lambda DNA maturation and packaging. Ann Rev Biochem. 1991;60:125–153. - PubMed
-
- Catalano CE. Viral Genome Packaging Machines: An Overview. In: Catalano CE, editor. Viral Genome Packaging Machines: Genetics, Structure, and Mechanism. Landes Bioscience; Georgetown, TX: 2005. pp. 1–4.
-
- Jardine PJ, Anderson D. DNA packaging in double-stranded DNA bacteriophages. In: Calendar R, editor. The Bacteriophages. Oxford Press; 2006. pp. 49–65.
-
- Feiss M, Catalano CE. Bacteriophage Lambda Terminase and the Mechanisms of Viral DNA Packaging. In: Catalano CE, editor. Viral Genome Packaging Machines: Genetics, Structure, and Mechanism. Landes Bioscience; Georgetown, TX: 2005. pp. 5–39.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

