Internalization and degradation of the glutamate transporter GLT-1 in response to phorbol ester
- PMID: 17919781
- PMCID: PMC2292111
- DOI: 10.1016/j.neuint.2007.08.020
Internalization and degradation of the glutamate transporter GLT-1 in response to phorbol ester
Abstract
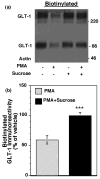
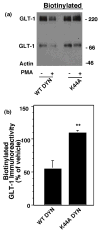
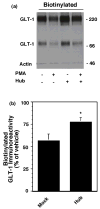
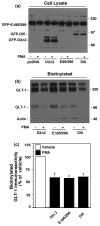
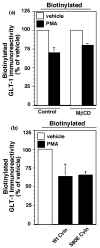
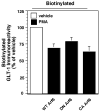
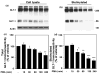
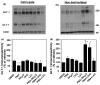
Activation of protein kinase C (PKC) decreases the activity and cell surface expression of the predominant forebrain glutamate transporter, GLT-1. In the present study, C6 glioma were used as a model system to define the mechanisms that contribute to this decrease in cell surface expression and to determine the fate of internalized transporter. As was previously observed, phorbol 12-myristate 13-acetate (PMA) caused a decrease in biotinylated GLT-1. This effect was blocked by sucrose or by co-expression with a dominant-negative variant of dynamin 1, and it was attenuated by co-expression with a dominant-negative variant of the clathrin heavy chain. Depletion of cholesterol with methyl-beta-cyclodextrin, co-expression with a dominant-negative caveolin-1 mutant (Cav1/S80E), co-expression with dominant-negative variants of Eps15 (epidermal-growth-factor receptor pathway substrate clone 15), or co-expression with dominant-negative Arf6 (T27N) had no effect on the PMA-induced loss of biotinylated GLT-1. Long-term treatment with PMA caused a time-dependent loss of biotinylated GLT-1 and decreased the levels of GLT-1 protein. Inhibitors of lysosomal degradation (chloroquine or ammonium chloride) or co-expression with a dominant-negative variant of a small GTPase implicated in trafficking to lysosomes (Rab7) prevented the PMA-induced decrease in protein and caused an intracellular accumulation of GLT-1. These results suggest that the PKC-induced redistribution of GLT-1 is dependent upon clathrin-mediated endocytosis. These studies identify a novel mechanism by which the levels of GLT-1 could be rapidly down-regulated via lysosomal degradation. The possibility that this mechanism may contribute to the loss of GLT-1 observed after acute insults to the CNS is discussed.
Figures
Similar articles
-
Evidence that protein kinase Calpha interacts with and regulates the glial glutamate transporter GLT-1.J Neurochem. 2005 Sep;94(5):1180-8. doi: 10.1111/j.1471-4159.2005.03330.x. Epub 2005 Jul 25. J Neurochem. 2005. PMID: 16045453
-
Glutamate transporter cluster formation in astrocytic processes regulates glutamate uptake activity.J Neurosci. 2004 Jul 14;24(28):6301-6. doi: 10.1523/JNEUROSCI.1404-04.2004. J Neurosci. 2004. PMID: 15254085 Free PMC article.
-
Protein kinase C activation decreases cell surface expression of the GLT-1 subtype of glutamate transporter. Requirement of a carboxyl-terminal domain and partial dependence on serine 486.J Biol Chem. 2002 Nov 29;277(48):45741-50. doi: 10.1074/jbc.M203771200. Epub 2002 Sep 24. J Biol Chem. 2002. PMID: 12324450
-
The Listeria protein internalin B mimics hepatocyte growth factor-induced receptor trafficking.Traffic. 2005 Jun;6(6):459-73. doi: 10.1111/j.1600-0854.2005.00290.x. Traffic. 2005. PMID: 15882443
-
Regulated trafficking of the human dopamine transporter. Clathrin-mediated internalization and lysosomal degradation in response to phorbol esters.J Biol Chem. 1999 Dec 10;274(50):35794-801. doi: 10.1074/jbc.274.50.35794. J Biol Chem. 1999. PMID: 10585462
Cited by
-
Traumatic Brain Injury Induces Alterations in Cortical Glutamate Uptake without a Reduction in Glutamate Transporter-1 Protein Expression.J Neurotrauma. 2017 Jan 1;34(1):220-234. doi: 10.1089/neu.2015.4372. Epub 2016 Jul 19. J Neurotrauma. 2017. PMID: 27312729 Free PMC article.
-
Extracellular microvesicles from astrocytes contain functional glutamate transporters: regulation by protein kinase C and cell activation.Front Cell Neurosci. 2013 Dec 10;7:251. doi: 10.3389/fncel.2013.00251. eCollection 2013. Front Cell Neurosci. 2013. PMID: 24368897 Free PMC article.
-
Inhibition of glycogen synthase kinase 3β activity with lithium prevents and attenuates paclitaxel-induced neuropathic pain.Neuroscience. 2013 Dec 19;254:301-11. doi: 10.1016/j.neuroscience.2013.09.033. Epub 2013 Sep 23. Neuroscience. 2013. PMID: 24070631 Free PMC article.
-
PKC-Dependent GlyT1 Ubiquitination Occurs Independent of Phosphorylation: Inespecificity in Lysine Selection for Ubiquitination.PLoS One. 2015 Sep 29;10(9):e0138897. doi: 10.1371/journal.pone.0138897. eCollection 2015. PLoS One. 2015. PMID: 26418248 Free PMC article.
-
The Regulation of GLT-1 Degradation Pathway by SIRT4.Neurochem Res. 2023 Sep;48(9):2847-2856. doi: 10.1007/s11064-023-03947-3. Epub 2023 May 13. Neurochem Res. 2023. PMID: 37178383
References
-
- Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nature Neurosci Rev. 2007;8:128–140. - PubMed
-
- Apparsundaram S, Schroeter S, Giovanetti E, Blakely RD. Acute regulation of norepinephrine transport:II. PKC-modulated surface expression of human norepinephrine transporter proteins. J Pharmacol Exp Ther. 1998;287:744–751. - PubMed
-
- Beckman ML, Quick MW. The ups and downs of neurotransmitter transporters. Neuroscientist. 2000;6:199–207.
-
- Benmerah A, Bayrou M, Cerf-Bensussan N, Dautry-Varsat A. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. Journal of Cell Science. 1999;112:1303–1311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

