Surface polyethylene glycol enhances substrate-mediated gene delivery by nonspecifically immobilized complexes
- PMID: 17920004
- PMCID: PMC2170460
- DOI: 10.1016/j.actbio.2007.08.008
Surface polyethylene glycol enhances substrate-mediated gene delivery by nonspecifically immobilized complexes
Abstract
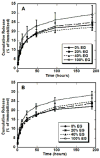
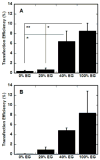
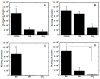
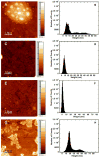
Substrate-mediated gene delivery describes the immobilization of gene therapy vectors to a biomaterial, which enhances gene transfer by exposing adhered cells to elevated DNA concentrations within the local microenvironment. Surface chemistry has been shown to affect transfection by nonspecifically immobilized complexes using self-assembled monolayers (SAMs) of alkanethiols on gold. In this report, SAMs were again used to provide a controlled surface to investigate whether the presence of oligo(ethylene glycol) (EG) groups in a SAM could affect complex morphology and enhance transfection. EG groups were included at percentages that did not affect cell adhesion. Nonspecific complex immobilization to SAMs containing combinations of EG- and carboxylic acid-terminated alkanethiols resulted in substantially greater transfection than surfaces containing no EG groups or SAMs composed of EG groups combined with other functional groups. Enhancement in transfection levels could not be attributed to complex binding densities or release profiles. Atomic force microscopy imaging of immobilized complexes revealed that EG groups within SAMs affected complex size and appearance and could indicate the ability of these surfaces to preserve complex morphology upon binding. The ability to control the morphology of the immobilized complexes and influence transfection levels through surface chemistry could be translated to scaffolds for gene delivery in tissue engineering and diagnostic applications.
Figures




Similar articles
-
Substrate-mediated delivery from self-assembled monolayers: effect of surface ionization, hydrophilicity, and patterning.Acta Biomater. 2005 Sep;1(5):511-22. doi: 10.1016/j.actbio.2005.05.004. Epub 2005 Jul 25. Acta Biomater. 2005. PMID: 16701831 Free PMC article.
-
Mechanism underlying bioinertness of self-assembled monolayers of oligo(ethyleneglycol)-terminated alkanethiols on gold: protein adsorption, platelet adhesion, and surface forces.Phys Chem Chem Phys. 2012 Aug 7;14(29):10196-206. doi: 10.1039/c2cp41236e. Epub 2012 Jun 21. Phys Chem Chem Phys. 2012. PMID: 22717889
-
Chain-length dependence of the protein and cell resistance of oligo(ethylene glycol)-terminated self-assembled monolayers on gold.J Biomed Mater Res. 2001 Sep 5;56(3):406-16. doi: 10.1002/1097-4636(20010905)56:3<406::aid-jbm1110>3.0.co;2-r. J Biomed Mater Res. 2001. PMID: 11372059
-
Substrate-mediated nucleic acid delivery from self-assembled monolayers.Trends Biotechnol. 2011 Mar;29(3):119-26. doi: 10.1016/j.tibtech.2010.11.005. Epub 2011 Jan 3. Trends Biotechnol. 2011. PMID: 21208672 Review.
-
Using self-assembled monolayers to understand the interactions of man-made surfaces with proteins and cells.Annu Rev Biophys Biomol Struct. 1996;25:55-78. doi: 10.1146/annurev.bb.25.060196.000415. Annu Rev Biophys Biomol Struct. 1996. PMID: 8800464 Review.
Cited by
-
Self-assembling peptide-lipoplexes for substrate-mediated gene delivery.Acta Biomater. 2009 Mar;5(3):903-12. doi: 10.1016/j.actbio.2008.10.003. Epub 2008 Oct 21. Acta Biomater. 2009. PMID: 18990615 Free PMC article.
-
Emerging links between surface nanotechnology and endocytosis: impact on nonviral gene delivery.Nano Today. 2010 Dec 1;5(6):553-569. doi: 10.1016/j.nantod.2010.10.007. Nano Today. 2010. PMID: 21383869 Free PMC article.
-
Biomaterial substrate modifications that influence cell-material interactions to prime cellular responses to nonviral gene delivery.Exp Biol Med (Maywood). 2019 Feb;244(2):100-113. doi: 10.1177/1535370218821060. Epub 2019 Jan 8. Exp Biol Med (Maywood). 2019. PMID: 30621454 Free PMC article. Review.
-
Fibrin hydrogels for non-viral vector delivery in vitro.J Control Release. 2009 Jun 5;136(2):148-54. doi: 10.1016/j.jconrel.2009.02.004. Epub 2009 Feb 20. J Control Release. 2009. PMID: 19232532 Free PMC article.
-
Multilayer mediated forward and patterned siRNA transfection using linear-PEI at extended N/P ratios.Acta Biomater. 2009 Jun;5(5):1474-88. doi: 10.1016/j.actbio.2009.01.004. Epub 2009 Jan 19. Acta Biomater. 2009. PMID: 19217360 Free PMC article.
References
-
- Niidome T, Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther. 2002;9:1647. - PubMed
-
- Herweijer H, Wolff JA. Progress and prospects: naked DNA gene transfer and therapy. Gene Ther. 2003;10:453. - PubMed
-
- Segura T, Shea LD. Materials for non-viral gene delivery. In: Stupp S, editor. Ann Rev Mater Sci. Vol. 31. 2001. p. 25. Annual Reviews.
-
- Ledley FD. Pharmaceutical approach to somatic gene therapy. Pharm Res. 1996;13:1595. - PubMed
-
- Pannier AK, Shea LD. Controlled release systems for DNA delivery. Mol Ther. 2004;10:19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

