Dilation and degradation of the brain extracellular matrix enhances penetration of infused polymer nanoparticles
- PMID: 17920047
- PMCID: PMC2169304
- DOI: 10.1016/j.brainres.2007.08.050
Dilation and degradation of the brain extracellular matrix enhances penetration of infused polymer nanoparticles
Abstract
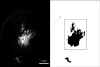
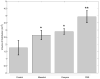
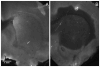
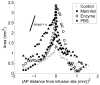
This study investigates methods of manipulating the brain extracellular matrix (ECM) to enhance the penetration of nanoparticle drug carriers in convection-enhanced delivery (CED). A probe was fabricated with two independent microfluidic channels to infuse, either simultaneously or sequentially, nanoparticles and ECM-modifying agents. Infusions were performed in the striatum of the normal rat brain. Monodisperse polystyrene particles with a diameter of 54 nm were used as a model nanoparticle system. Because the size of these particles is comparable to the effective pore size of the ECM, their transport may be significantly hindered compared with the transport of low molecular weight molecules. To enhance the transport of the infused nanoparticles, we attempted to increase the effective pore size of the ECM by two methods: dilating the extracellular space and degrading selected constituents of the ECM. Two methods of dilating the extracellular space were investigated: co-infusion of nanoparticles and a hyperosmolar solution of mannitol, and pre-infusion of an isotonic buffer solution followed by infusion of nanoparticles. These treatments resulted in an increase in the nanoparticle distribution volume of 51% and 123%, respectively. To degrade hyaluronan, a primary structural component of the brain ECM, a pre-infusion of hyaluronidase (20,000 U/mL) was followed after 30 min by infusion of nanoparticles. This treatment resulted in an increase in the nanoparticle distribution of 64%. Our results suggest that both dilation and enzymatic digestion can be incorporated into CED protocols to enhance nanoparticle penetration.
Figures



Similar articles
-
Real-time imaging of perivascular transport of nanoparticles during convection-enhanced delivery in the rat cortex.Ann Biomed Eng. 2012 Feb;40(2):292-303. doi: 10.1007/s10439-011-0440-0. Epub 2011 Oct 19. Ann Biomed Eng. 2012. PMID: 22009318 Free PMC article.
-
Strategies to enhance the distribution of nanotherapeutics in the brain.J Control Release. 2017 Dec 10;267:232-239. doi: 10.1016/j.jconrel.2017.07.028. Epub 2017 Jul 21. J Control Release. 2017. PMID: 28739449 Free PMC article.
-
Distribution of polymer nanoparticles by convection-enhanced delivery to brain tumors.J Control Release. 2016 Jun 28;232:103-12. doi: 10.1016/j.jconrel.2016.04.006. Epub 2016 Apr 8. J Control Release. 2016. PMID: 27063424 Free PMC article.
-
Hyaluronidase overcomes the extracellular matrix barrier to enhance local drug delivery.Eur J Pharm Biopharm. 2024 Oct;203:114474. doi: 10.1016/j.ejpb.2024.114474. Epub 2024 Aug 25. Eur J Pharm Biopharm. 2024. PMID: 39191305 Review.
-
Digesting a Path Forward: The Utility of Collagenase Tumor Treatment for Improved Drug Delivery.Mol Pharm. 2018 Jun 4;15(6):2069-2083. doi: 10.1021/acs.molpharmaceut.8b00319. Epub 2018 May 16. Mol Pharm. 2018. PMID: 29767984 Free PMC article. Review.
Cited by
-
Evaluating the safety profile of focused ultrasound and microbubble-mediated treatments to increase blood-brain barrier permeability.Expert Opin Drug Deliv. 2019 Feb;16(2):129-142. doi: 10.1080/17425247.2019.1567490. Epub 2019 Jan 29. Expert Opin Drug Deliv. 2019. PMID: 30628455 Free PMC article. Review.
-
Numerical study of nanofluid infusion in deformable tissues for hyperthermia cancer treatments.Med Biol Eng Comput. 2011 Nov;49(11):1233-40. doi: 10.1007/s11517-011-0819-y. Epub 2011 Aug 14. Med Biol Eng Comput. 2011. PMID: 21842423
-
In vivo evaluation of intracellular drug-nanocarriers infused into intracranial tumours by convection-enhanced delivery: distribution and radiosensitisation efficacy.J Neurooncol. 2010 Apr;97(2):195-205. doi: 10.1007/s11060-009-0012-4. Epub 2009 Sep 22. J Neurooncol. 2010. PMID: 19768659
-
Nanotherapeutic systems for local treatment of brain tumors.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018 Jan;10(1):10.1002/wnan.1479. doi: 10.1002/wnan.1479. Epub 2017 May 24. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018. PMID: 28544801 Free PMC article. Review.
-
Convection-enhanced delivery of camptothecin-loaded polymer nanoparticles for treatment of intracranial tumors.Drug Deliv Transl Res. 2011 Feb 1;1(1):34-42. doi: 10.1007/s13346-010-0001-3. Drug Deliv Transl Res. 2011. PMID: 21691426 Free PMC article.
References
-
- Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, Cunningham J, Budinger TF, Harvey-White J. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;162:2–14. - PubMed
-
- Bourgoin C, Emiliani C, Kremer EJ, Gelot A, Tancini B, Gravel RA, Drugan C, Orlaccio A, Poenaru L, Caillaud C. Widespread distribution of β-hexosaminidase activity in the brain of a Sandhoff mouse model after coinjection of adenoviral vector and mannitol. Gene Therapy. 2003;10:1841–1849. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

