Site-directed alkylation of LacY: effect of the proton electrochemical gradient
- PMID: 17920075
- PMCID: PMC2224895
- DOI: 10.1016/j.jmb.2007.09.006
Site-directed alkylation of LacY: effect of the proton electrochemical gradient
Abstract
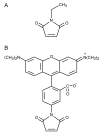
Previous N-ethylmaleimide-labeling studies show that ligand binding increases the reactivity of single-Cys mutants located predominantly on the periplasmic side of LacY and decreases reactivity of mutants located for the most part of the cytoplasmic side. Thus, sugar binding appears to induce opening of a periplasmic pathway with closing of the cytoplasmic cavity resulting in alternative access of the sugar-binding site to either side of the membrane. Here we describe the use of a fluorescent alkylating reagent that reproduces the previous observations with respect to sugar binding. We then show that generation of an H(+) electrochemical gradient (Delta(mu (H)+), interior negative) increases the reactivity of single-Cys mutants on the periplasmic side of the sugar-binding site and in the putative hydrophilic pathway. The results suggest that Delta(mu (H)+), like sugar, acts to increase the probability of opening on the periplasmic side of LacY.
Figures

Similar articles
-
Site-directed alkylation and the alternating access model for LacY.Proc Natl Acad Sci U S A. 2007 Jan 9;104(2):491-4. doi: 10.1073/pnas.0609968104. Epub 2006 Dec 15. Proc Natl Acad Sci U S A. 2007. PMID: 17172438 Free PMC article.
-
The Cys154-->Gly mutation in LacY causes constitutive opening of the hydrophilic periplasmic pathway.J Mol Biol. 2008 Jun 13;379(4):695-703. doi: 10.1016/j.jmb.2008.04.015. Epub 2008 Apr 11. J Mol Biol. 2008. PMID: 18485365 Free PMC article.
-
Residues gating the periplasmic pathway of LacY.J Mol Biol. 2009 Nov 27;394(2):219-25. doi: 10.1016/j.jmb.2009.09.043. Epub 2009 Sep 23. J Mol Biol. 2009. PMID: 19781551 Free PMC article.
-
The alternating access transport mechanism in LacY.J Membr Biol. 2011 Jan;239(1-2):85-93. doi: 10.1007/s00232-010-9327-5. Epub 2010 Dec 16. J Membr Biol. 2011. PMID: 21161516 Free PMC article. Review.
-
It takes two to tango: The dance of the permease.J Gen Physiol. 2019 Jul 1;151(7):878-886. doi: 10.1085/jgp.201912377. Epub 2019 May 30. J Gen Physiol. 2019. PMID: 31147449 Free PMC article. Review.
Cited by
-
Evidence for an intermediate conformational state of LacY.Proc Natl Acad Sci U S A. 2012 Mar 20;109(12):E698-704. doi: 10.1073/pnas.1201107109. Epub 2012 Feb 21. Proc Natl Acad Sci U S A. 2012. PMID: 22355148 Free PMC article.
-
Trp replacements for tightly interacting Gly-Gly pairs in LacY stabilize an outward-facing conformation.Proc Natl Acad Sci U S A. 2013 May 28;110(22):8876-81. doi: 10.1073/pnas.1306849110. Epub 2013 May 13. Proc Natl Acad Sci U S A. 2013. PMID: 23671103 Free PMC article.
-
Role of the irreplaceable residues in the LacY alternating access mechanism.Proc Natl Acad Sci U S A. 2012 Jul 31;109(31):12438-42. doi: 10.1073/pnas.1210684109. Epub 2012 Jul 16. Proc Natl Acad Sci U S A. 2012. PMID: 22802658 Free PMC article.
-
Sugar binding induces the same global conformational change in purified LacY as in the native bacterial membrane.Proc Natl Acad Sci U S A. 2010 May 25;107(21):9903-8. doi: 10.1073/pnas.1004515107. Epub 2010 May 10. Proc Natl Acad Sci U S A. 2010. PMID: 20457922 Free PMC article.
-
The periplasmic cavity of LacY mutant Cys154→Gly: how open is open?Biochemistry. 2013 Sep 17;52(37):6568-74. doi: 10.1021/bi401026d. Epub 2013 Aug 30. Biochemistry. 2013. PMID: 23962108 Free PMC article.
References
-
- Kaback HR. Structure and mechanism of the lactose permease. C R Biol. 2005;328:557–67. - PubMed
-
- Viitanen P, Newman MJ, Foster DL, Wilson TH, Kaback HR. Purification, reconstitution, and characterization of the lac permease of Escherichia coli. Methods Enzymol. 1986;125:429–452. - PubMed
-
- Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

