Selective induction of cell-mediated immunity and protection of rhesus macaques from chronic SHIV(KU2) infection by prophylactic vaccination with a conserved HIV-1 envelope peptide-cocktail
- PMID: 17920095
- PMCID: PMC2196441
- DOI: 10.1016/j.virol.2007.08.022
Selective induction of cell-mediated immunity and protection of rhesus macaques from chronic SHIV(KU2) infection by prophylactic vaccination with a conserved HIV-1 envelope peptide-cocktail
Abstract
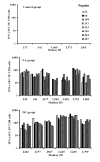
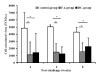
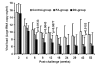
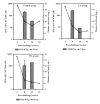
Infection of Indian-origin rhesus macaques by the simian human immunodeficiency virus (SHIV) is considered to be a suitable preclinical model for directly testing efficacy of vaccine candidates based on the HIV-1 envelope. We used this model for prophylactic vaccination with a peptide-cocktail comprised of highly conserved HIV-1 envelope sequences immunogenic/antigenic in macaques and humans. Separate groups of macaques were immunized with the peptide-cocktail by intravenous and subcutaneous routes using autologous dendritic cells (DC) and Freund's adjuvant, respectively. The vaccine elicited antigen specific IFN-gamma-producing cells and T-cell proliferation, but not HIV-neutralizing antibodies. The vaccinated animals also exhibited efficient cross-clade cytolytic activity against target cells expressing envelope proteins corresponding to HIV-1 strains representative of multiple clades that increased after intravenous challenge with pathogenic SHIV(KU2). Virus-neutralizing antibodies were either undetectable or present only transiently at low levels in the control as well as vaccinated monkeys after infection. Significant control of plasma viremia leading to undetectable levels was achieved in majority of vaccinated monkeys compared to mock-vaccinated controls. Monkeys vaccinated with the peptide-cocktail using autologous DC, compared to Freund's adjuvant, and the mock-vaccinated animals, showed significantly higher IFN-gamma production, higher levels of vaccine-specific IFN-gamma producing CD4(+) cells and significant control of plasma viremia. These results support DC-based vaccine delivery and the utility of the conserved HIV-1 envelope peptide-cocktail, capable of priming strong cell-mediated immunity, for potential inclusion in HIV vaccination strategies.
Figures











Similar articles
-
Protection against chronic infection and AIDS by an HIV envelope peptide-cocktail vaccine in a pathogenic SHIV-rhesus model.Vaccine. 2001 Dec 12;20(5-6):813-25. doi: 10.1016/s0264-410x(01)00408-x. Vaccine. 2001. PMID: 11738745
-
Vaccination with the Conserved Caveolin-1 Binding Motif in Human Immunodeficiency Virus Type 1 Glycoprotein gp41 Delays the Onset of Viral Infection and Provides Partial Protection in Simian/Human Immunodeficiency Virus-Challenged Cynomolgus Macaques.J Virol. 2018 Aug 29;92(18):e00370-18. doi: 10.1128/JVI.00370-18. Print 2018 Sep 15. J Virol. 2018. PMID: 29976675 Free PMC article.
-
Vaccination of rhesus macaques with recombinant Mycobacterium bovis bacillus Calmette-Guérin Env V3 elicits neutralizing antibody-mediated protection against simian-human immunodeficiency virus with a homologous but not a heterologous V3 motif.J Virol. 2005 Feb;79(3):1452-62. doi: 10.1128/JVI.79.3.1452-1462.2005. J Virol. 2005. PMID: 15650171 Free PMC article.
-
Protection of neonatal macaques against experimental SHIV infection by human neutralizing monoclonal antibodies.Transfus Clin Biol. 2001 Aug;8(4):350-8. doi: 10.1016/s1246-7820(01)00187-2. Transfus Clin Biol. 2001. PMID: 11642027 Review.
-
Structure-based vaccine design in HIV: blind men and the elephant?Curr Pharm Des. 2010;16(33):3744-53. doi: 10.2174/138161210794079173. Curr Pharm Des. 2010. PMID: 21128885 Free PMC article. Review.
Cited by
-
Safety and immunogenicity of a CTL multiepitope peptide vaccine for HIV with or without GM-CSF in a phase I trial.Vaccine. 2009 Jan 7;27(2):243-9. doi: 10.1016/j.vaccine.2008.10.051. Epub 2008 Nov 7. Vaccine. 2009. PMID: 18996425 Free PMC article. Clinical Trial.
-
T cell-based strategies for HIV-1 vaccines.Hum Vaccin Immunother. 2020 Mar 3;16(3):713-722. doi: 10.1080/21645515.2019.1666957. Epub 2019 Oct 25. Hum Vaccin Immunother. 2020. PMID: 31584318 Free PMC article.
-
Functional impairment of central memory CD4 T cells is a potential early prognostic marker for changing viral load in SHIV-infected rhesus macaques.PLoS One. 2011;6(5):e19607. doi: 10.1371/journal.pone.0019607. Epub 2011 May 13. PLoS One. 2011. PMID: 21602924 Free PMC article.
-
Protection against Mucosal SHIV Challenge by Peptide and Helper-Dependent Adenovirus Vaccines.Viruses. 2009 Dec 1;1(3):920. doi: 10.3390/v1030920. Viruses. 2009. PMID: 20107521 Free PMC article.
-
Cell targeting and immunostimulatory properties of a novel Fcγ-receptor-independent agonistic anti-CD40 antibody in rhesus macaques.Cell Mol Life Sci. 2023 Jun 23;80(7):189. doi: 10.1007/s00018-023-04828-2. Cell Mol Life Sci. 2023. PMID: 37353664 Free PMC article.
References
-
- Amara RR, Villinger F, Altman JD, Lydy SL, O’Neil SP, Staprans SI, Montefiori DC, Herndon GS, Wyatt MA, Candido NL, Kozyr PL, Earl JM, Smith HL, Ma BD, Grimm ML, Hulsey J Miller, McClure HM, McNicholl JM, Moss B, Robinson HL. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;92:69–74. - PubMed
-
- Baba TW, Liska V, Lehmann RH, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou T, Ruprecht RM. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nature Med. 2000;6:200–206. - PubMed
-
- Banchereau J, Paczesny S, Blanco P, Bennett L, Pascual V, fay J, Palucka AK. Dendritic cells: controllers of the immune system and a new promise for immunotherapy. Ann N Y Acad Sci. 2003;987:180–187. - PubMed
-
- Barouch DH, Fu TM, Montefiori DC, Lewis MG, Shiver JW, Letvin NL. Vaccine-elicited immune responses prevent clinical AIDS in SHIV (89.6P)-infected rhesus monkeys. Immunol Lett. 2001;79:57–61. - PubMed
-
- Barouch DH, Kunstaman J, Juroda MJ, Schmitz JE, Santra S, Peyerl FW, Krivulka GR, Beaudry K, Lifton MA, Gotgone DA, Montefiori DC, Lewis MG, Wolinsky SM, Letvin NL. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature. 2002;415:335–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

